or start from open source methods. Learn more about OneLab softwareUse OneLab
Automated NucleoBond Xtra Midiprep

This advanced method offers an end-to-end solution developed around an advanced application, a specialized kit, or an analytical system. It delivers complete functional, ready-to-use protocols that are qualitatively and quantitatively assessed for consistency, executability, and repeatability, usually without needing to make any changes. It operates per batch size and is scalable to accommodate changing requirements.
Overview
NucleoBond Xtra Midi – Unmatched Performance, now Automated on Andrew+
An ever-growing range of biochemical applications requires medium to large amounts of plasmid DNA free of contamination with salts and residual bacterial components. With NucleoBond Xtra yields of up to 500 μg of ultrapure plasmid DNA can be obtained based on reliable and well-established anion exchange chromatography. NucleoBond Xtra Midi kits contain enlarged columns, which lead to lower silica resin beds. This in turn enables the faster flow of lysate and buffers through the columns. Specially designed column filters are included for convenient and time-saving clarification of bacterial lysates. The column filters are supplied pre-inserted in the NucleoBond Xtra Columns and allow parallel clarification of bacterial lysate and loading onto the column. Their large, structured surface leads to high filter flow rates and minimized risk of clogging.
Principle / Procedure
Transformed (plasmid-containing) bacterial cells are lysed by an optimized set of buffers. After equilibration of the NucleoBond Xtra Column and NucleoBond Xtra Column Filter, the entire lysate is loaded by gravity flow and simultaneously cleared by the NucleoBond Xtra Column Filter. Plasmid DNA is bound to the NucleoBond Xtra Silica Resin. After efficient washing, the plasmid DNA is eluted and desalted by magnetic beads.
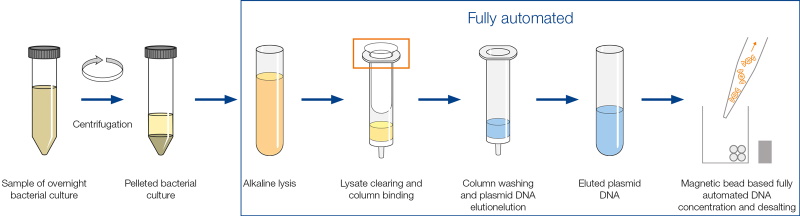
The automated workflow of the MN NucleoBond Xtra Midi kit on the Andrew+ liquid handler dramatically reduces the hands-on time needed for a plasmid midi prep. The Andrew+ robot equipped with the appropriate holders (Dominos) performs all steps, from lysate clarification to elution and concentration of pure plasmid DNA, freeing up your valuable time for doing actual science. Andrew+ empowers scientists to get reproducible data – and the accompanying software, OneLab, offers the most intuitive solution for protocol design and experiment traceability. Andrew+ provides a seamless transition from time-consuming manual pipetting to error-free and easily programmable robotics.
Reliably High-Quality Plasmid DNA with NucleoBond Xtra Midi
NucleoBond Xtra Midi and the Andrew+ combine the high purity and reliability of anion-exchange technology with the user-friendliness of a fully automated system. NucleoBond Xtra Midi delivers a reliable yield over a span of sample amounts (Figure 1). It is therefore ideally suited for obtaining large amounts of pure plasmid DNA suitable for transfection and in vivo experiments.
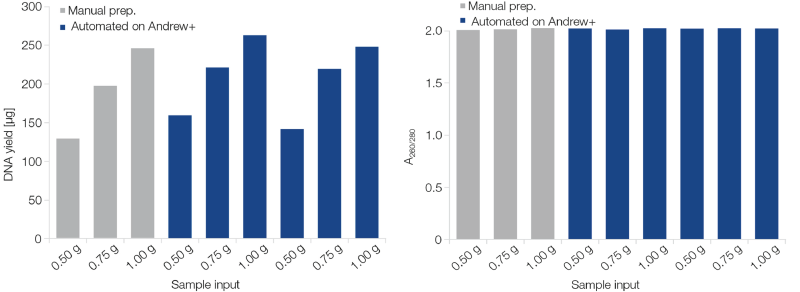
Figure 1: Excelling plasmid yield and purity – manual or automated preparation. Different amounts of E. coli culture pellets expressing a high-copy plasmid were subjected to the NucleoBond Xtra Midi procedure, manually (one set of samples) or automated on the Andrew+ robot (two sets of samples). The workflow includes automated desalting and concentration of plasmid DNA with NucleoMag Desalting Beads. Both manual and automated preps deliver excellent results regarding plasmid conformation, yield, and purity (A260/280).
Reduce Sample-to-Sample Variation by Automating NucleoBond Xtra Midi
Automated processing of NucleoBond Xtra Midi on the Andrew+ delivers consistently satisfactory yields and minimizes variations in performance usually caused by small deviations during manual processing (Figure 2).
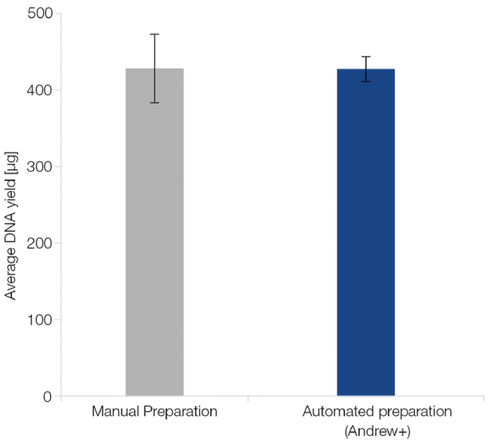
Figure 2: Consistent results with minimal fluctuations. 0.8 g of E. coli culture pellets expressing a high copy plasmid were subjected to the NucleoBond Xtra Midi procedure, manually (blue bars), or automated on the Andrew+ robot (orange bars; n=6 for each method). Desalting and concentration of plasmid DNA were conducted by the robot using NucleoMag Desalting Beads. While both methods deliver high amounts of pure plasmid DNA, the yields of the manual preparations show a higher standard variation (±45.6 μg) than those of the automated method (±15.8 μg). In conclusion, automating the NucleoBond Xtra Midi preparation on Andrew+ significantly reduces sample-to-sample variations in yield.
Andrew+ Visual Deck Layouts for Automated NucleoBond Xtra Midiprep OneLab Protocols
◼️ Automated NucleoBond Xtra Midiprep, 3 Samples | Transfection-grade

Figure 3: Configuration of Andrew+ Dominos and connected devices, i.e., the 50mL Tube Magnet+, for the Automated NucleoBond Xtra Midiprep, 3-Sample protocol. For a fully automated protocol, Andrew+ uses a column gripper to move columns hosted by the NucleoBond Xtra Midi Column Domino from/to the 50mL Tube Magnet+.
◼️ Automated NucleoBond Xtra Midiprep, 6 Samples | Transfection-grade & Endotoxin-free (EF)
▶ Protocol Execution without Tip Refill
If you are running the 6-sample protocol (transfection grade or EF) without the tip refill option, we recommend selecting a NARROW deck layout for optimal column trajectory (Figure 4).
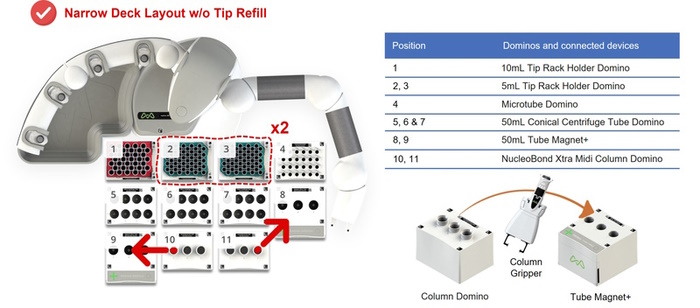
Figure 4: Narrow deck configuration of Andrew+ Dominos and connected devices, i.e., the 50mL Tube Magnet+, for the Automated NucleoBond Xtra Midiprep, 6-Sample protocol without tip refill. For a fully automated protocol, Andrew+ uses a column gripper to move columns hosted by the NucleoBond Xtra Midi Column Domino from/to the 50mL Tube Magnet+. The two red arrows show the trajectory of the columns.
▶ Protocol Execution with Tip Refill
If you are running the 6-sample protocol (transfection grade or EF) with the tip refill option, we recommend selecting a WIDE deck layout for optimal column trajectory (Figure 5).
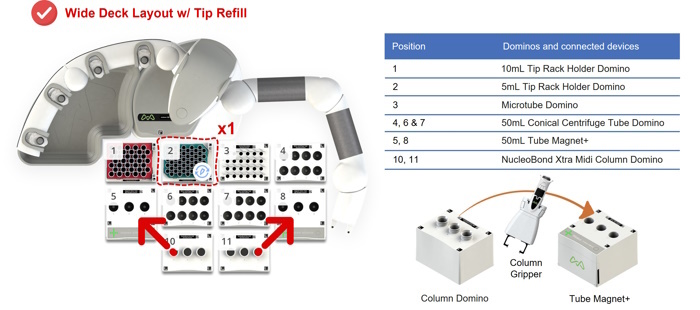
Figure 5: Wide deck configuration of Andrew+ Dominos and connected devices, i.e., the 50mL Tube Magnet+, for the Automated NucleoBond Xtra Midiprep, 6-Sample protocol with tip refill. For a fully automated protocol, Andrew+ uses a column gripper to move columns hosted by the NucleoBond Xtra Midi Column Domino from/to the 50mL Tube Magnet+. The two red arrows show the trajectory of the columns.
Andrew+ deck configuration and pipette selection are similar for both the "Automated NucleoBond Xtra Midi" (transfection-grade) protocol and the "Automated NucleoBond Xtra Midi EF" (endotoxin-free) protocol. However, kit chemistry and buffer volumes are different for the transfection-grade and endotoxin-free procedures. Endotoxin values can vary with bacterial strain, plasmid backbone, and bacterial pellet size. A laminar flow hood is not required for purifying endotoxin-free plasmid DNA.
Protocol Specifications
▶ NucleoBond Xtra Midiprep for purification of transfection-grade plasmid DNA
- Number of samples per run | 6 samples
- Recommended pellet wet weight | 0.5 - 1.0 g per sample or ODV = 250 - 550
- Approx. time | 3 hours
- Hands-on step | Removal of NucleoBond Xtra Midi Column Filters after lysate clarification (approx. 5 min)
- Tip consumption | 54x 5 mL tips and 34x 10 mL tips
▶ NucleoBond Xtra Midiprep EF for purification of endotoxin-free plasmid DNA
- Number of samples per run | 6 samples
- Recommended pellet wet weight | 0.5 - 1.0 g per sample or ODV = 250 - 550
- Approx. time | 3h 10min
- Hands-on step | Removal of NucleoBond Xtra Midi Column Filters after lysate clarification (approx. 5 min)
- Tip consumption | 56x 5 mL tips and 34x 10 mL tips
ORDERING INFORMATION
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
➤ Automated NucleoBond Xtra Midiprep, 3 Samples – Andrew+
- 1x 5mL Tip Rack Holder Domino | p/n 186009599
- 1x 10mL Tip Rack Holder Domino | p/n 186010098
- 2x 50mL Conical Centrifuge Tube Domino | p/n 186009614
- 1x Microtube Domino | p/n 186009601
- 1x NucleoBond Xtra Midi Column Domino | p/n 186010156
- 1x 50mL Tube Magnet+ | p/n 176004851
- Andrew Alliance Bluetooth Column Gripper | p/n 186010179
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 100 – 5,000 μL | p/n 186009608
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 100 – 10,000 μL | p/n 186009767
┉
Selection 1, with standard Tips:
- Sartorius, Optifit Tips, 100-5,000 μL (x28) | p/n 700013291
- Sartorius, Optifit Tips, 100-10,000 μL (x17) | p/n 700013301
OR Selection 2, with Filter Tips:
- Sartorius, Safetyspace(TM) Filter Tips, 100-5,000 μL (x28) | p/n LH-795001F
- Sartorius, Safetyspace(TM) Filter Tips are unavailable in 10 mL volume. Use standard, Sartorius Optifit Tips, 100-10,000 μL (x17)
➤ Automated NucleoBond Xtra Midiprep, 6 Samples – Andrew+
- 2x 5mL Tip Rack Holder Domino | p/n 186009599
- 1x 10mL Tip Rack Holder Domino | p/n 186010098
- 3x 50mL Conical Centrifuge Tube Domino | p/n 186009614
- 1x Microtube Domino | p/n 186009601
- 2x NucleoBond Xtra Midi Column Domino | p/n 186010156
- 2x 50mL Tube Magnet+ | p/n 176004851
- Andrew Alliance Bluetooth Column Gripper | p/n 186010179
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 100 – 5,000 μL | p/n 186009608
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 100 – 10,000 μL | p/n 186009767
┉
Selection 1, with standard Tips:
- Sartorius, Optifit Tips, 100-5,000 μL (x54) | p/n 700013291
- Sartorius, Optifit Tips, 100-10,000 μL (x34) | p/n 700013301
OR Selection 2, with Filter Tips:
- Sartorius, Safetyspace(TM) Filter Tips, 100-5,000 μL (x54) | p/n LH-795001F
- Sartorius, Safetyspace(TM) Filter Tips are unavailable in 10 mL volume. Use standard, Sartorius Optifit Tips, 100-10,000 μL (x34)
➤ Automated NucleoBond Xtra Midiprep EF, 6 Samples – Andrew+
- 2x 5mL Tip Rack Holder Domino | p/n 186009599
- 1x 10mL Tip Rack Holder Domino | p/n 186010098
- 3x 50mL Conical Centrifuge Tube Domino | p/n 186009614
- 1x Microtube Domino | p/n 186009601
- 2x NucleoBond Xtra Midi Column Domino | p/n 186010156
- 2x 50mL Tube Magnet+ | p/n 176004851
- Andrew Alliance Bluetooth Column Gripper | p/n 186010179
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 100 – 5,000 μL | p/n 186009608
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 100 – 10,000 μL | p/n 186009767
- Sartorius, Optifit Tips, 100-5,000 μL (x56) | p/n 700013291
- Sartorius, Optifit Tips, 100-10,000 μL (x34) | p/n 700013301
Application Kits
Kit for the Automated NucleoBond Xtra Midi Protocol
- NucleoBond Xtra Midi kit ⎯ Anion exchange-based plasmid midi prep kit (10, 50, 100 preps); NucleoBond® Xtra Midi columns included | REF: 740410.10/.50/.100
Kit for the Automated NucleoBond Xtra Midi EF Protocol
- NucleoBond Xtra Midi EF kit ⎯ Endotoxin-free, anion exchange-based plasmid midi prep kit (10, 50 preps); NucleoBond® Xtra Midi columns included | REF: 740420.10/.50
Reagents & Chemicals (Not provided in NucleoBond Xtra Midi kits)
- NucleoMag Desalting Beads ⎯ Magnetic beads for desalting of anion exchange eluates (50 preps) | REF: 744410.50
Recommended Consumables
The automated workflow of the MN NucleoBond® Xtra Midi kit on the Andrew+ liquid handler dramatically reduces the hands-on time needed for a plasmid midi prep. The Andrew+ robot with required Dominos (labware holders), performs all the steps of the prep, from lysate clarification down to elution of pure plasmid DNA prep, freeing up your valuable time for doing actual science.
Check Out MACHEREY-NAGEL Protocol Information Guides
- Protocol Information Andrew Alliance NucleoBond Xtra Midiprep, 3 Samples
- Protocol Information Andrew Alliance NucleoBond Xtra Midiprep, 6 Samples
- Protocol Information Andrew Alliance NucleoBond Xtra Midiprep, 6 Samples, Endotoxin-free
Download NucleoBond® Xtra Midi User Manual for Plasmid DNA Purification
Download NucleoBond® Xtra Midi EF User Manual for Endotoxin-free Plasmid DNA Purification
Protocols



Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 