or start from open source methods. Learn more about OneLab softwareUse OneLab
Valita®Titer Antibody Quantitation
This example method provides a freely adjustable framework for measuring the adaptability of the OneLab environment to workflows from different application fields. It helps to understand various nuances of the code-free, universal protocol designer and provides general indications as to the feasibility of a project. Scripts generally require fine adjustment to correct for variables and support specific labware implementation.
Overview
IgG Quantification in Drug Development
Biologics are a very promising source of medicines for treating various diseases including inflammatory diseases, cancers, infections, and autoimmune diseases. Among biologic drugs, monoclonal antibodies (mAb) and mAb variants represent the largest and fastest-growing group of biopharmaceuticals because of their ability to target diseases specifically and to achieve high efficacy with a low risk of toxicity. During late-stage development and cell culture-based manufacturing and control of recombinant IgG antibodies, concentrations of IgG in a large number of samples must be reliably measured to assess and identify the ideal conditions that ensure stable and adequate cell-specific productivity and product yield. Measurement of IgG concentration is also essential in basic and clinical research.
Current methods allowing for detection and measurement of IgG binding and concentration include ELISA, Protein A-HPLC analysis, SPR-based analysis (Biacore™ system), Bio-Layer Interferometry (Octet® System), and HTRF (homogenous time-resolved fluorescence). These methods are usually costly, laborious, and require relatively long sample preparation and analysis times.
The Valita®Titer assay uses fluorescence polarization for rapid and high-throughput quantitation of IgG antibody titer in liquid samples (e.g. cell culture media or supernatant) across a functional range from 2.5 to 80 mg/L. It provides a simple and cost-effective microplate-based solution for accurate measurement of IgG concentrations without a need for complex sample preparation or purification steps. The Valita®Titer assay enables not only the quantification of IgG subclasses (IgG1 to 4), but also various IgG types such as IgG kappa, IgG lambda, IgG Fc-fusion proteins, and IgG bispecific antibodies.
Fluorescence Polarization
Fluorescence polarization (FP) allows for the characterization and quantification of interactions between large proteins (e.g. antibodies) and small fluorophore-conjugated molecules (e.g. peptides) based on the change in polarization of emitted light upon excitation of the fluorophore with plane-polarized light. The degree of polarization of emitted light is inversely related to the rate of rotation, which in turn depends on the molecular size. Small molecules rotate faster in solution than large molecules. The more the fluorescent molecule rotates during the time between excitation and emission of light, the more the light is depolarized. Therefore, when a fluorescently labeled peptide is unbound, the emitted light exhibits low polarization due to the rapid rotation in the solution (Figure 1 top). Upon binding to a specific antibody, the degree of polarization increases due to the slower tumbling of the bound peptide (Figure 1 bottom).
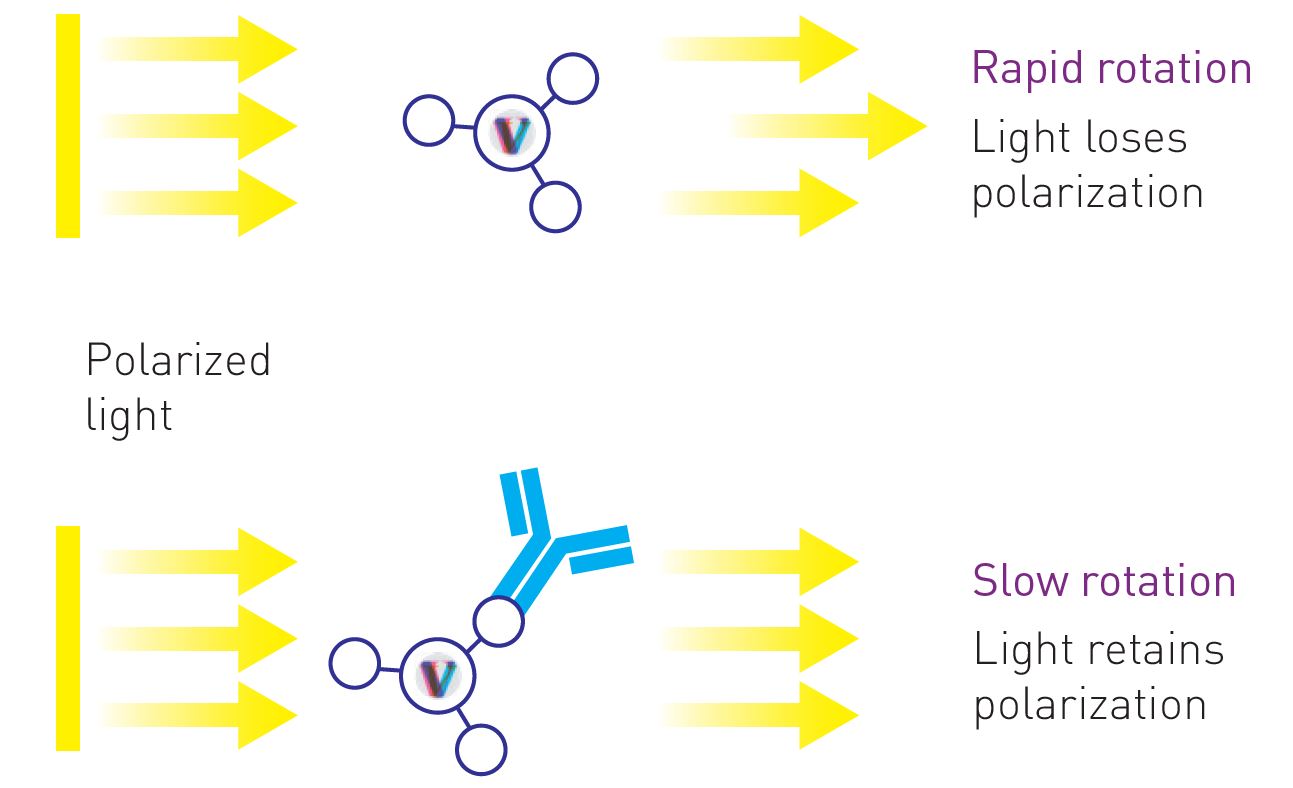
Figure 1: Schematic representation showing the effect of the molecular size on the rotation rate and subsequently the degree of polarization of emitted light. When a fluorescently labeled IgG-binding peptide is unbound (top), it rotates much faster in solution and thus depolarizes the light more than when it is bound to an IgG (bottom). The change in polarization caused by molecular rotation is used to measure the amount of IgG antibodies in the sample.
Valita®Titer Assay Principle
The Valita®Titer assay relies on the detection of IgG-Fc binding to a fluorescently labeled derivative of protein G, a highly specific IgG-binding protein used as a probe, by measuring the change in polarization of emitted light caused by molecular rotation. When the fluorescently labeled protein G is unbound, it tumbles rapidly in solution and depolarises the emitted light. The binding of protein G to the target IgG induces an increase in the polarization value due to the high molecular weight of the IgG-protein G complex formed. The magnitude to which the emitted light moves from the excitation plane (vertical) to a perpendicular plane (horizontal) - i.e. the change in polarization between excitation and emission light - is a function of the rotation rate of the fluorescent probe. The change in polarization is used to determine the degree of protein G binding and thereby the amount of IgG of interest in the solution. FP is typically measured by exciting the solution with plane-polarized light and measuring the intensity of light emitted in two planes, one parallel to the exciting light (polarized proportion) and the other perpendicular to the exciting light (depolarized portion). Quantitatively, FP is defined as the difference between the emission light intensity parallel and perpendicular to the excitation light plane normalized by the total fluorescence emission intensity. FP is expressed as a normalized difference of these two intensities, which is typically in millipolarization units (mP).
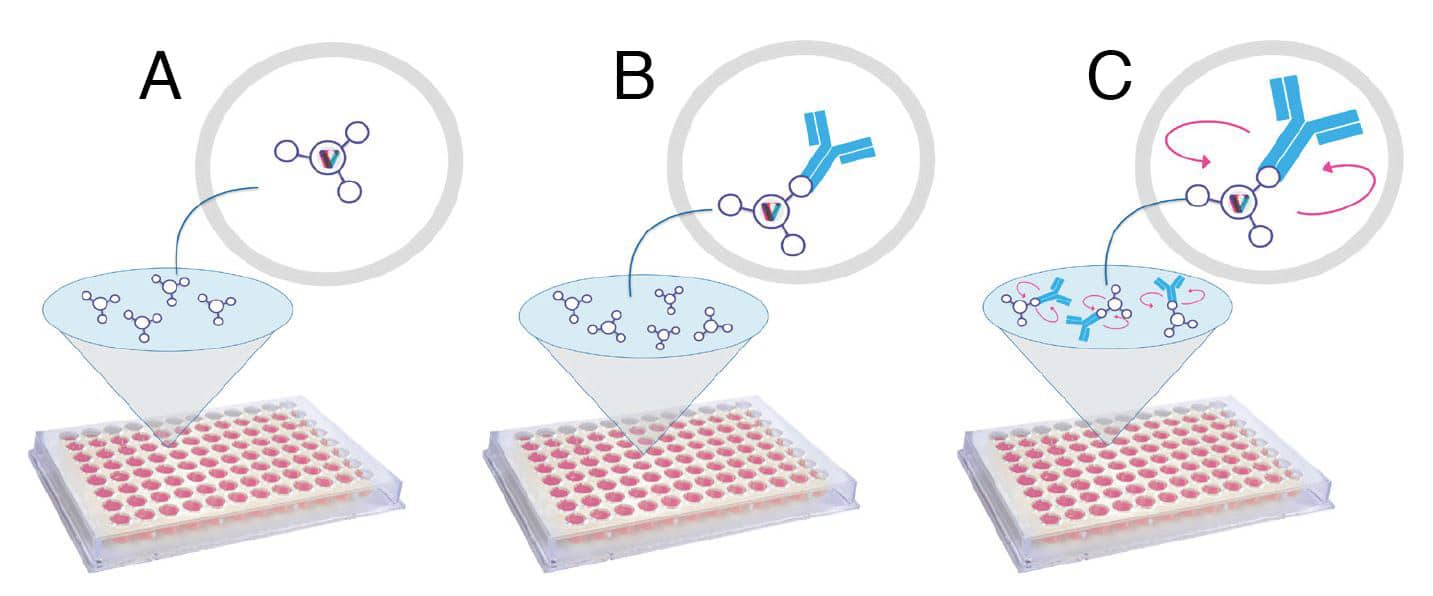
Figure 2: Illustration of the three key steps of the Valita®Titer assay for the quantitative measurement of IgG antibodies using FP. A) Wells of the 96-well microtiter plate are pre-coated with a fluorescently labeled IgG-specific protein G. The protein G is reconstituted by the addition of the ValitaMAb buffer B) After loading samples, IgG binds to the protein G, which induces a change in polarization of emitted light. C) IgG concentration is measured using FP, which is inversely related to the rate of rotation of the fluorescent probe. The fluorescent complex IgG-protein G rotates slower than the unbound protein G, resulting in an increase in polarization value.
Experimental Procedure
The Valita®Titer assay Kit comprises the Valita®Titer plate and the Valita™MAb Buffer. The microtiter plate wells are pre-coated with FITC-labeled protein G (Figure 2A). Following the Valita®Titer assay instructions, kit components, test samples, and IgG standards are brought to room temperature before starting the experiment. Test samples and IgG standards are diluted as needed in fresh cell growth media. 60μL of Valita™MAb buffer is added to each well of the microtiter 96-well plate to reconstitute the fluorescent probe. 60μL of each standard or test sample is then added to the appropriate wells and mixed. For best results, all standards and test samples are analyzed in triplicate. The plate is sealed and then incubated on a flat surface in the dark for 30 minutes at room temperature. At the molecular level, IgG antibodies in the test sample will bind to the fluorescent probes, which results in a change in polarization of emitted light in the assay well of the microtitre plate (Figure 2B and C). The plate is read on a configured FP plate reader, such as the BMG PHERAstar microplate reader to measure FP intensities. Data are analyzed using the Valita™APP analysis software provided by ValitaCell. As a result, an eight-point standard curve is generated from IgG standard samples with known concentrations and the corresponding change in polarization of light emitted (Figure 3). The concentration of IgG antibodies in each test sample is finally calculated based on a comparison to the standard curve.
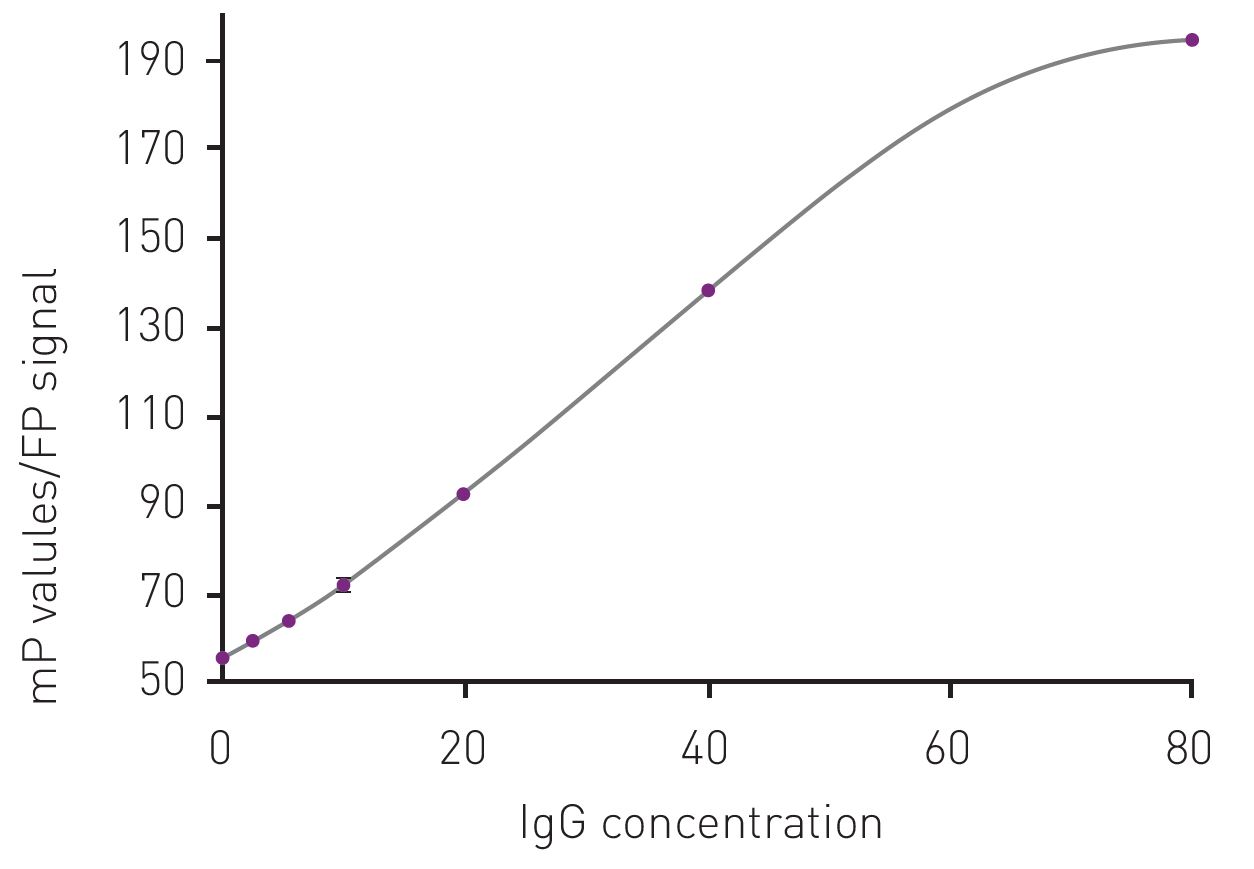
Figure 3: Example of a standard curve for the determination of IgG concentration using the measured FP signal. The standard curve is prepared in the concentration range of 0-80mg/L of IgG antibody. The concentration of IgG in mg/L is plotted versus the polarization signal expressed in millipolarization units (mP).
Automation of Valita®Titer Assay Using Andrew+
Here below are the working deck layouts demonstrating the automation of the Valita®Titer assay using the Andrew+ Pipetting Robot.
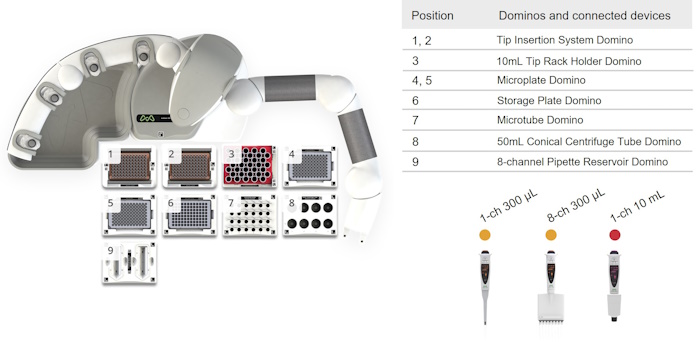
Figure 4: Andrew+ deck layout for the protocol "ValitaTiter Assay, 24 Samples in Plate, Pipette Mixing".

Figure 5: Andrew+ deck layout for the protocol "ValitaTiter Assay, 24 Samples in Tubes, Pipette Mixing".
Protocol Specifications
▶ ValitaTiter Assay, 24 Samples in Plate, Pipette mixing – Andrew+
- Estimated time of execution | 39min 24sec
- Tip consumption | 134x 5-350 μL tips and 1x 100-10,000 μL tips
▶ ValitaTiter Assay, 24 Samples in Tubes, Pipette Mixing – Andrew+
- Estimated time of execution | 1h 0min 55sec
- Tip consumption | 230x 5-350 μL tips and 1x 100-10,000 μL tips
For support for using or editing this protocol, please contact support@valitacell.com.
ORDERING INFORMATION
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
➤ ValitaTiter Assay, 24 Samples in Plate, Pipette mixing – Andrew+
- Microtube Domino | p/n 186009601
- 2x Microplate Domino | p/n 186009600
- Storage Plate Domino | p/n 186009596
- 8-Channel Pipette Reservoir Domino | p/n 186009613
- 50mL Conical Centrifuge Tube Domino | p/n 186009614
- 2x Tip Insertion System Domino | p/n 186009612
- 10mL Tip Rack Holder Domino | p/n 186010098
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 10 mL | p/n 186009767
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Sartorius, Optifit Tips, 5-350 μL (x134) | p/n 700013297
- Sartorius, Optifit Tips, 100-10,000 μL (x1) | p/n 700013301
➤ ValitaTiter Assay, 24 Samples in Tubes, Pipette mixing – Andrew+
- 2x Microtube Domino | p/n 186009601
- 2x Microplate Domino | p/n 186009600
- 8-Channel Pipette Reservoir Domino | p/n 186009613
- 50mL Conical Centrifuge Tube Domino | p/n 186009614
- 3x Tip Insertion System Domino | p/n 186009612
- 10mL Tip Rack Holder Domino | p/n 186010098
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 10 mL | p/n 186009767
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Sartorius, Optifit Tips, 5-350 μL (x230) | p/n 700013297
- Sartorius, Optifit Tips, 100-10,000 μL (x1) | p/n 700013301
Learn more about Valitacell’s Quantum Technology and Key Applications
Explore the Valita®Titer Assay, for Rapid, High-throughput IgG Quantification
Protocols

Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 