or start from open source methods. Learn more about OneLab softwareUse OneLab
Standard Curve Generation
This basic method provides the core methodology for translating a workflow into OneLab-executable script(s) as an attempt to fully or semi-automate a specific procedure. It demonstrates the benefits of automation and highlights OneLab capabilities and best practices to promote solution adoption, helping transition from manual to a more automated approach. It can be used alone or serves as a building block for a more complex workflow and is easily adaptable to users' requirements.
Overview
Addressing the Challenges in Sample Preparation for Standard Curve Generation
The preparation of samples is often the most time-consuming, but critical step, for the laboratory analyst. Any errors made in the sample preparation step will follow through the rest of the analysis potentially causing high variability in performance and in extreme cases failed analysis. In addition, the development, optimization, and execution of these assays can prove to be time-consuming and difficult to transfer between scientists and laboratories (1).
The creation of calibration standards and quality control (QC) samples is a requirement in all quantitative analyses. These samples are generated using the basic technique of sequential (or serial) dilution of the analyte(s) from a concentrated solution(s). While often not difficult, preparation of these samples, is repetitive and time-consuming, requiring consistent preparation to ensure the reliable performance of the analytical method. This makes it ideal for incorporating lab automation for this task. This allows the analyst to do other tasks, streamlines the sample preparation process, reduces the potential of human error, and ensures consistent analytical method performance.
Automation Benefits
- Accurate, precise, and robust analytic quantification performance
- Ease-of-use and time savings
- Cost savings through increased efficiency and failure reduction
- Less repetitive pipetting
- Automated method transferability
◼️ PROTOCOL 1 | Standard Curve Preparation - Nitrosamine Impurities | Andrew+
The following work highlights the development of an automated sample preparation method using the Andrew+ Pipetting Robot for the LC-MS quantification of N-nitrosamine impurities and demonstrates its performance. Due to their carcinogenicity and incidence in a variety of commodities and pharmaceuticals, robust and reliable sample preparation and analysis for their routine quantification during and after drug development and manufacturing are required.
ORDERING INFORMATION
Consumables & Systems
- Waters 0.7 mL 96-round well collection plate | p/n 186005837
- Agilent 6-column reagent reservoir | p/n 201284-100
- Fisherbrand™ Premium 1.5 mL microtube | p/n 11926955
- Waters Pre-slit silicone/PTFE cap mat for 96-well plate, 7 mm round wells | p/n 186006332
- ACQUITY UPLC HSS T3 column | p/n 186003539
- ACQUITY UPLC I-Class PLUS system
- Xevo TQ-XS Tandem Quadrupole Mass Spectrometer
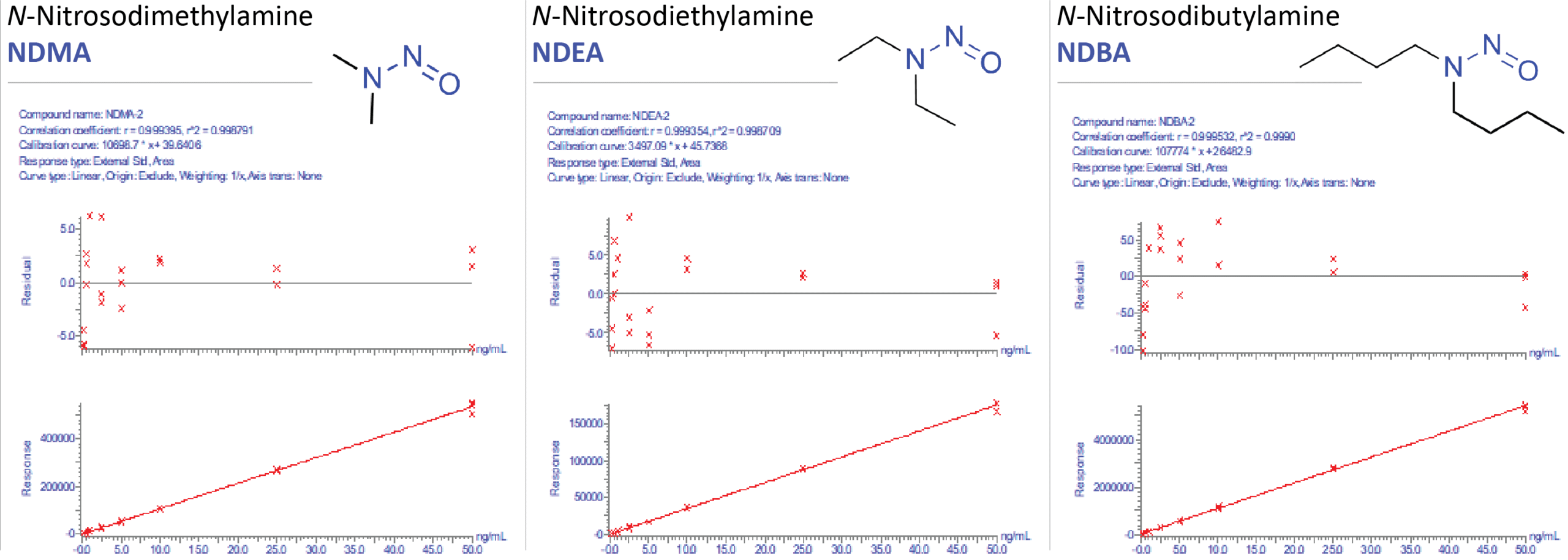
Figure 1: Representative Nitrosamine Impurity Standard Calibration Curves (NDMA, NDEA, and NDBA), using Andrew+ Pipetting Robot for Calibration Curve Generation.
◼️ PROTOCOL 2 | Final Dilution - Carnitine by LC-MS/MS | Andrew+
This protocol was developed to generate a final 10 times dilution (1:10) of samples for the analysis of carnitine by LC-MS/MS. It is usually performed by 1 to 10 mL manual dilution in a test tube. The Andrew+ Pipetting Robot automates this dilution by transferring 150 μL sample solutions and 1350 μL diluent (ACN/water) into 2 mL vials and mixing.
ORDERING INFORMATION
Andrew+ System Components: Dominos & Electronic Pipettes
- Tip Insertion System Domino | p/n 186009612
- 5mL Tip Rack Holder Domino | p/n 186009599
- 2mL HPLC Vial Rack Domino | p/n 186010091
- Microtube Domino | p/n 186009601
- 50mL Conical Centrifuge Tube Domino | p/n 186009614
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 5000 µL | p/n 186009608
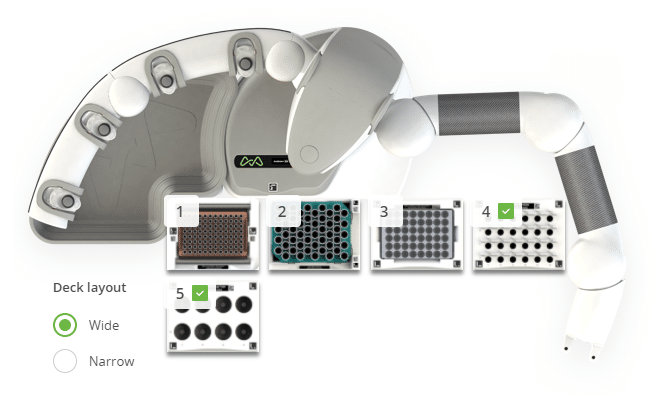
Figure 2: Andrew+ deck configuration for the "Final Dilution - Carnitine by LC-MS/MS" protocol. Domino positions on the Andrew+ working deck: [1] Tip Insertion System Domino, [2] 5mL Tip Rack Holder Domino, [3] 2mL HPLC Vial Rack Domino, [4] Microtube Domino, [5] 50mL Conical Centrifuge Tube Domino.
◼️ PROTOCOL 3 | Final Dilution - Cysteine & Methionine by LC-FLR | Andrew+
This protocol was developed to generate a final 50 times dilution (1:50) of samples for the analysis of cysteine and methionine by LC-FLR (Liquid Chromatography-Fluorescence Detection). It is usually performed by 2 to 100 mL manual dilution in a volumetric flask. The Andrew+ Pipetting Robot automates this dilution by transferring 10 μL sample solutions and 490 μL diluent (water) into HPLC filter vials in Acquity sample plate and post-dilution mixing.
ORDERING INFORMATION
Andrew+ System Components: Dominos & Electronic Pipettes
- 2x Tip Insertion System Domino | p/n 186009612
- 2mL HPLC Vial Rack Domino | p/n 186010091
- 29mL Glass Culture Tube Domino | p/n 186010194
- 50mL Conical Centrifuge Tube Domino | p/n 186009614
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 10 μL | p/n 186009769
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
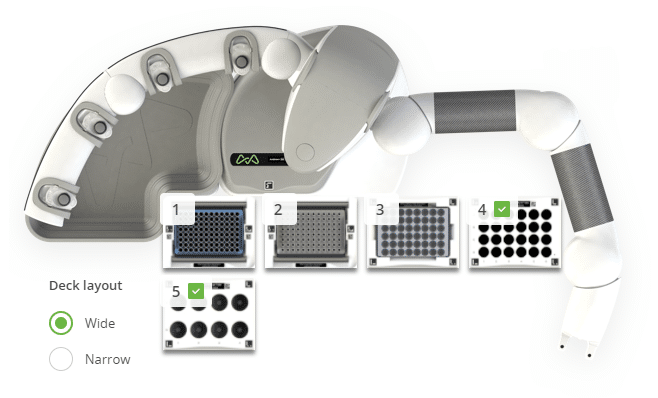
Figure 3: Andrew+ deck configuration for the "Final Dilution - Cysteine and Methionine by LC-FLR" protocol. Domino positions on the Andrew+ working deck: [1,2] Tip Insertion System Domino, [3] 2mL HPLC Vial Rack Domino, [4] 29mL Glass Culture Tube Domino, [5] 50mL Conical Centrifuge Tube Domino.
◼️ PROTOCOL 4 | Standard Curve Preparation - Galactose by IC-ED | Andrew+
This protocol was developed for the galactose standard solution preparation at concentrations of 10,000 μg/mL, 5,000 μg/mL, 2,000 μg/mL, 500 μg/mL, 200 μg/mL, 50 μg/mL, 20 μg/mL. These galactose standard solutions are used in IC-ED (Ion Chromatography-Electrochemical Detection) analysis. The Andrew+ Pipetting Robot automates a serial dilution of 1-3 mL of standard solutions with diluent (25% MeOH in water) in 50 mL centrifuge tubes and post-dilution mixing.
ORDERING INFORMATION
Andrew+ System Components: Dominos & Electronic Pipettes
- Tip Insertion System Domino | p/n 186009612
- 5mL Tip Rack Holder Domino | p/n 186009599
- 50mL Conical Centrifuge Tube Domino | p/n 186009614
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 5000 μL | p/n 186009608

Figure 4: Andrew+ deck configuration for the "Standard Curve Preparation - Galactose by IC-ED" protocol. Domino positions on the Andrew+ working deck: [1] Tip Insertion System Domino, [2] 5mL Tip Rack Holder Domino, [3] 50mL Conical Centrifuge Tube Domino.
◼️ PROTOCOL 5 | Standard Curve Preparation - Vitamin A by LC-FLR | Andrew+
This protocol was developed for the vitamin A (retinol) standard solution preparation for LC-FLR (Liquid Chromatography-Fluorescence Detection) analysis. The Andrew+ Pipetting Robot automates a serial dilution of 1-10 mL of standard solutions with diluent (hexane) in 50 mL centrifuge tubes and post-dilution mixing.
ORDERING INFORMATION
Andrew+ System Components: Dominos & Electronic Pipettes
- Tip Insertion System Domino | p/n 186009612
- 10mL Tip Rack Holder Domino | p/n 186010098
- 50mL Conical Centrifuge Tube Domino | p/n 186009614
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 10,000 μL | p/n 186009767

Figure 5: Andrew+ deck configuration for the "Standard Curve Preparation - Vitamin A by LC-FLR" protocol. Domino positions on the Andrew+ working deck: [2] Tip Insertion System Domino; [2] 10mL Tip Rack Holder Domino, [3] 50mL Conical Centrifuge Tube Domino.
(1) Christler, A., Felföldi, E., Mosor, M., Sauer, D., Walch, N., Dürauer, A., & Jungbauer, A. (2020). Semi-automation of process analytics reduces operator effect. Bioprocess and Biosystems Engineering, 43(5), 753–764.
Protocols

Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 