or start from open source methods. Learn more about OneLab softwareUse OneLab
SARS-CoV-2 LC-MS Kit (RUO)
This advanced method offers an end-to-end solution developed around an advanced application, a specialized kit, or an analytical system. It delivers complete functional, ready-to-use protocols that are qualitatively and quantitatively assessed for consistency, executability, and repeatability, usually without needing to make any changes. It operates per batch size and is scalable to accommodate changing requirements.
Overview
Analysis of SARS-CoV-2 peptides for a greater understanding of SARS-CoV-2
There is a critical demand for time-saving and accurate sample preparation and analysis in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) research. SARS-CoV-2 is the virus responsible for the coronavirus disease 2019 (COVID-19), which has been the subject of much research due to the global pandemic. One of the most abundant viral proteins in SARS-CoV-2 is the Nucleocapsid (NCAP) protein (Figure 1), which makes it a useful target for quantitation and analysis. Waters has developed a SARS-CoV-2 LC-MS kit that takes advantage of UPLC-MS/MS for faster analysis. This solution allows for the analysis of three proteotypic peptides AYN, NPA, and ADE from the SARS-CoV-2 NCAP protein.
To enable fast and accurate sample preparation, the SARS-CoV-2 LC-MS kit includes:
- Waters SARS-CoV-2 LC-MS Enrichment Set (RUO) - antibody magnetic beads
- Waters SARS-CoV-2 LC-MS Reagent Set (RUO)
- Calibrator and Internal Standard Set (RUO)
- System Suitability Solution Set (RUO)
- Analytical column and in-line filter
Analysis of NCAP protein and challenges
The analysis of large complex molecules like proteins and peptides can be challenging and requires robust sample preparation and sensitive analytical methods. In this sample preparation workflow, the signature peptides are enriched with anti-peptide antibodies to greatly improve multiple reaction monitoring (MRM) sensitivity for Mass Spectrometry. The workflow also uses stable isotope-labeled synthetic peptides as true internal standards for consistent quantitation.
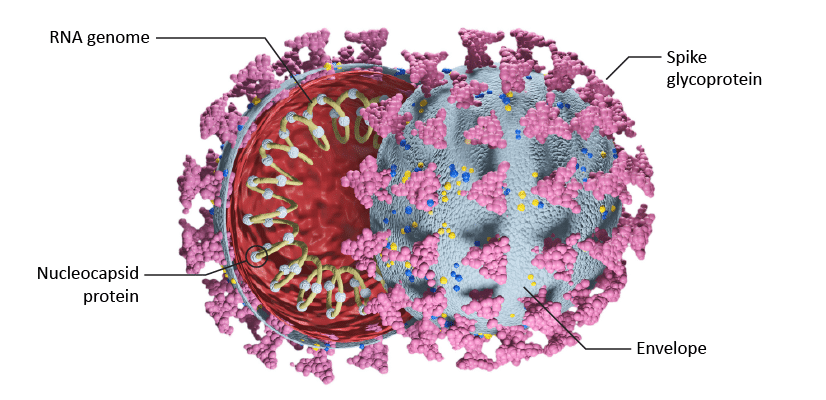
Figure 1: Cross-sectional view of SARS-CoV-2 structure showing RNA and Nucleocapsid protein.
Preparation of NCAP protein using SARS-CoV-2 LC-MS Kit
Experimental Procedure
This workflow describes the denaturation, digestion, and enrichment with UPLC-MS/MS analysis of peptides AYN, NPA, and ADE using the SARS-CoV-2 LC-MS Kit. It is for the quantitative detection of tryptic peptides for research use only and not diagnostic testing.
Sample containing SARS-CoV-2 NCAP protein initially goes through denaturation and digestion steps to unfold the protein and generate the tryptic peptides (Figure 2). NCAP is denatured by adding 20 μL of denaturant and then mixed well on a plate shaker at 1500 rpm for 30 seconds before sealing the plate for incubation at 56°C for 15 minutes. The plate seal is removed to add 20 μL Trypsin Solution and then mixed well on a plate shaker at 1500 rpm for 30 seconds. The plate is then sealed and incubated on a plate shaker at 37°C for 30 minutes. The plate seal is removed to add 20 μL of TLCK solution to each well to inhibit trypsin activity. The plate is mixed on a shaker at 1500 rpm for 5 minutes at room temperature. Then 20 μL of the stable isotope-labeled (SIL) peptide mixture is added before mixing on a shaker at 1500 rpm for 30 seconds at room temperature. The SIL will be used as a true internal standard in the sample preparation for quantitation.
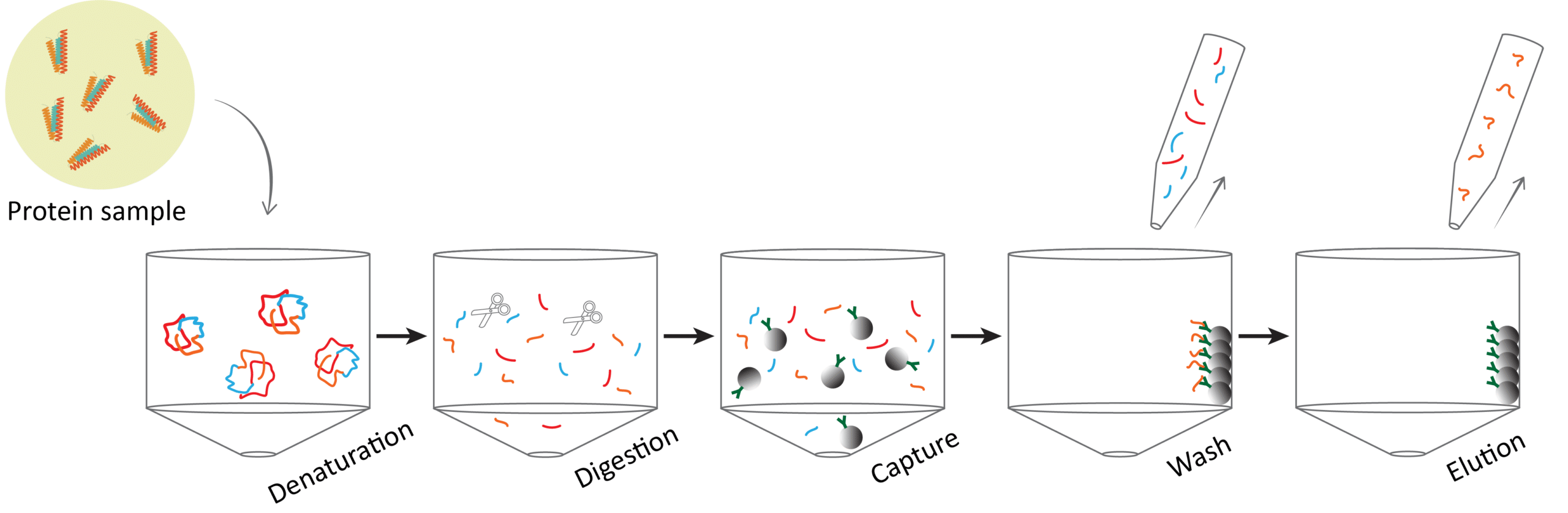
Figure 2: SARS-CoV-2 LC-MS sample preparation workflow.
The next step in the process involves the three tryptic SARS-CoV-2 peptides being captured in the sample and enriched by antibody reagents cross-linked with magnetic beads (Figure 2). The use of magnetic beads allows for the selective capture of peptides resulting in a highly purified sample of peptide analytes. To each well of the plate, a 30 μL volume of resuspended antibody-coupled magnetic beads is added. The magnetic bead mixture will require mixing at a minimum of every four samples during addition to the plate. The plate is shaken at 1500 rpm for 10 seconds until the beads are fully resuspended and then continues to shake for an hour at 1200 rpm.
Once the capture process is complete, the sample is washed whilst a magnetic force retains the antibody-captured peptides in the sample plate (Figure 2). The plate is placed on a magnetic plate for a minute until the beads are drawn to one side. All the liquid is removed whilst avoiding the beads and collected in a second plate for further processing if required. Beads are then washed with the addition of 150 μL of wash buffer and shaken at 1500 rpm for 10 seconds at room temperature to resuspend the beads. The shaking speed is then dropped to 1200 rpm for a further 20 seconds at room temperature. The collection plate is replaced on the magnetic plate to draw the beads to one side again. The liquid is drawn out and the wash step is repeated a second time to ensure a highly purified sample.
Finally, peptides are eluted as a very clean and concentrated sample (Figure 2). To each well, a 50 μL volume of elution buffer is added and the plate is shaken at 1500 rpm for 10 seconds at room temperature until the beads are resuspended before continuing on at 1200 rpm for 5 minutes. This step allows bound peptides to be released into the buffer ready for collection. The sample plate is placed on the magnetic plate once again to draw the magnetic beads aside. The eluent is removed and transferred into another plate where it is sealed and centrifuged, ready for UPLC-MS/MS analysis.
Automation on the Andrew+ Liquid Handling Robot
The increase in demand for testing of SARS-CoV-2 in research has lead scientists to implement options such as automation to access results swiftly and at a higher throughput. This allows the user to focus on other tasks such as processing results while more samples are being prepared. The SARS CoV-2 LC-MS kit has been created in conjunction with Andrew+ automation to provide all the necessary reagents and consumables required with a protocol in one straightforward solution. This sample preparation method delivers highly purified samples which also cuts down LC run times further facilitating high-throughput sample processing.
The SARS-CoV-2 protocol employs the same process used in manual sample preparation with adaptations for use with Andrew+ connected devices (Figure 3).
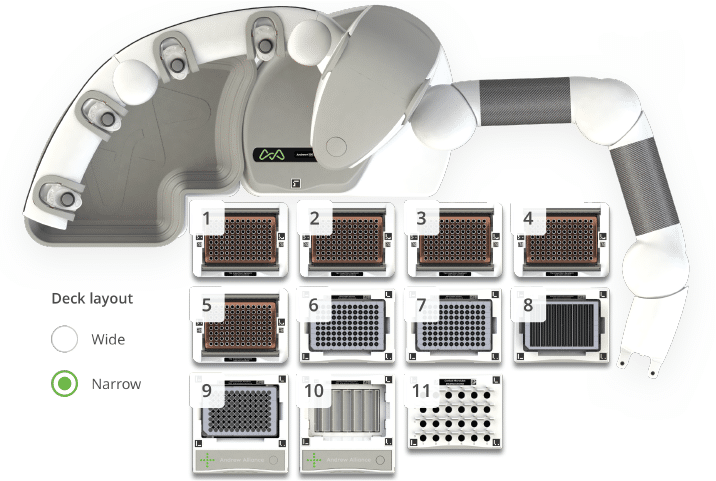
Figure 3: Andrew+ Domino configuration for the automated SARS-CoV-2 LC-MS 96 sample protocol. Domino/Device positions on the Andrew+ working deck: [1-5] Tip Insertion System Domino, [6&7] Storage Plate Domino, [8] Deepwell Microplate Domino, [9] Microplate Shaker+, [10] Deepwell Magnet+, [11] Microtube Domino.
Considerations
1- Wash Step
- The wash step should be completed as quickly as possible taking no longer than 20 minutes. If running large sample batches washes should be completed in subsets.
- The flow-through collected from the wash step can be collected for further extraction and/or analysis for research purposes using a collection plate.
2- Enrichment
- It is important that the magnetic bead solution does not fall out of suspension. The solution should be mixed with every 4 samples.
3- Removal of Wash and Elution Solutions
- Due to the magnetic beads within the well, it is important to carefully pipette to one side so as not to remove the beads.
ORDERING INFORMATION
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
➤ SARS-CoV-2 LC-MS Sample Prep, 96 Samples – Andrew+
- Deepwell Microplate Domino | p/n 186009597
- Microtube Domino | p/n 186009601
- 2x Storage Plate Domino | p/n 186009596
- 5x Tip Insertion System Domino | p/n 186009612
- Microplate Shaker+ | p/n 176004577
- Deepwell Magnet+ | p/n 176004854
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Sartorius, Optifit Tips, 5-350 μL (x427) | p/n 700013297
Application Kits
Application kits are designed for running up to 96 sample batches.
Application Kits & Standards
- Waters SARS-CoV-2 LC-MS Starter Kit (RUO) | p/n 176004946
- Waters SARS-CoV-2 LC-MS Re-Order Kit (RUO) | p/n 176004947
- Waters SARS-CoV-2 LC-MS Sample Preparation and Reagent Kit (RUO) | p/n 176004948
- Waters SARS-CoV-2 LC-MS System Suitability Solution Set (RUO) | p/n 176004949
Individual Kit Components
- Waters SARS-CoV-2 LC-MS System Suitability Solution (RUO) | p/n 186010232
- ACQUITY PREMIER Peptide BEH C18 Column, 300Å, 1.7 μm, 2.1 x 30 mm by Waters | p/n 186010094
- ACQUITY PREMIER Peptide BEH C18 Column, 300Å, 1.7 μm, 2.1 x 50 mm by Waters | p/n 186009493
- Formic Acid Mobile Phase Additive by Waters | p/n 186006691
- Kit, ACQUITY Column In-Line Filter by Waters | p/n 205000343
Reagents & Chemicals (Not provided in SARS-CoV-2 LC-MS kits)
- 0.1 M Hydrochloric acid solution by Supelco/Merck | p/n 2104 or equivalent
- Acetonitrile, ULC/MS-grade by Biosolve | p/n 012041 or equivalent
- Viral transport media by VWR International | p/n LIOF26490
- 18.2 MΩ Water or equivalent
Recommended Consumables
- Fisherbrand™ Premium 1.5 mL microtube | p/n 11926955
- Axygen® 12-well reservoir, 12-channel trough | p/n RES-MW12-HP
Check out Waters SARS-CoV-2 LCMS (RUO) Kit for more information
Protocols

Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 