or start from open source methods. Learn more about OneLab softwareUse OneLab
PyroSmart NextGen® Recombinant Cascade Reagent (rCR) for Endotoxin Detection

This advanced method offers an end-to-end solution developed around an advanced application, a specialized kit, or an analytical system. It delivers complete functional, ready-to-use protocols that are qualitatively and quantitatively assessed for consistency, executability, and repeatability, usually without needing to make any changes. It operates per batch size and is scalable to accommodate changing requirements.
Overview
Use of the Andrew+ Robot and OneLab to Automate Liquid Handling for Bacterial Endotoxin Testing
PyroSmart NextGen® is a recombinant protein-based Bacterial Endotoxin Test (BET) (1,2,4,5) used to detect endotoxin. The assay utilizes a complex series of enzymatic reactions to quantify endotoxin. This series of enzymatic reactions is sometimes referred to as the Limulus amebocyte lysate (LAL) cascade (2,3) (Figure 1). Under standard conditions, this assay is extremely sensitive. It can detect minute quantities of E. coli endotoxin down to 0.005EU/mL (EU: endotoxin units per milliliter) range (i.e., sub nanogram (10-9 g/mL) (2). Endotoxins, or lipopolysaccharides, are amphipathic molecules derived from the cell surface of gram-negative bacteria such as E. coli (Figure 2) (6,7). These molecules belong to a group of substances called pyrogens because they have been observed to cause a rise in body temperature. Avoidance of an unintended immune response resulting from endotoxins necessitates testing. Any injectable drug or medical device is assayed for endotoxins to ensure safety. For example, any vaccine or biologic must be tested for the presence of endotoxin in the final formulation.
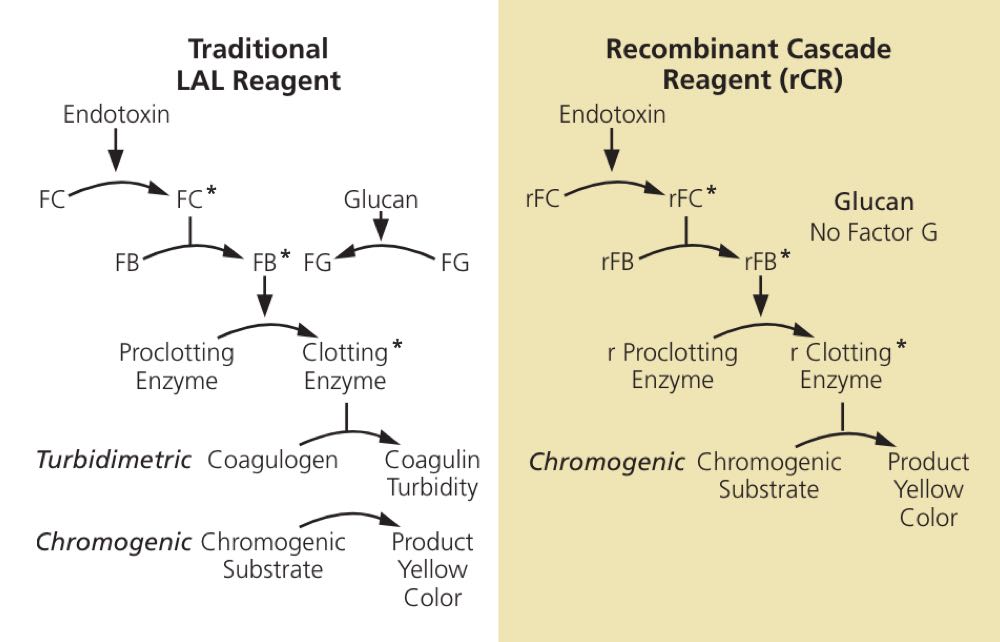
Figure 1: Traditional and Recombinant LAL Cascades. In the traditional cascade on the left, an extract from horseshoe crabs is used. This contains protein zymogens sensitive to both endotoxins and glucans. On the right-hand side, the same cascade for endotoxin detection is derived from recombinant proteins. This reagent does not have the proteins present in the biological extract (FG) that can lead to cross-reactivity with (1→3)-β-D-glucans. Abbreviations: FC-Factor C, FB-Factor B, FG-Factor G, asterisks denote activated enzyme forms. See reference 4 for more details.
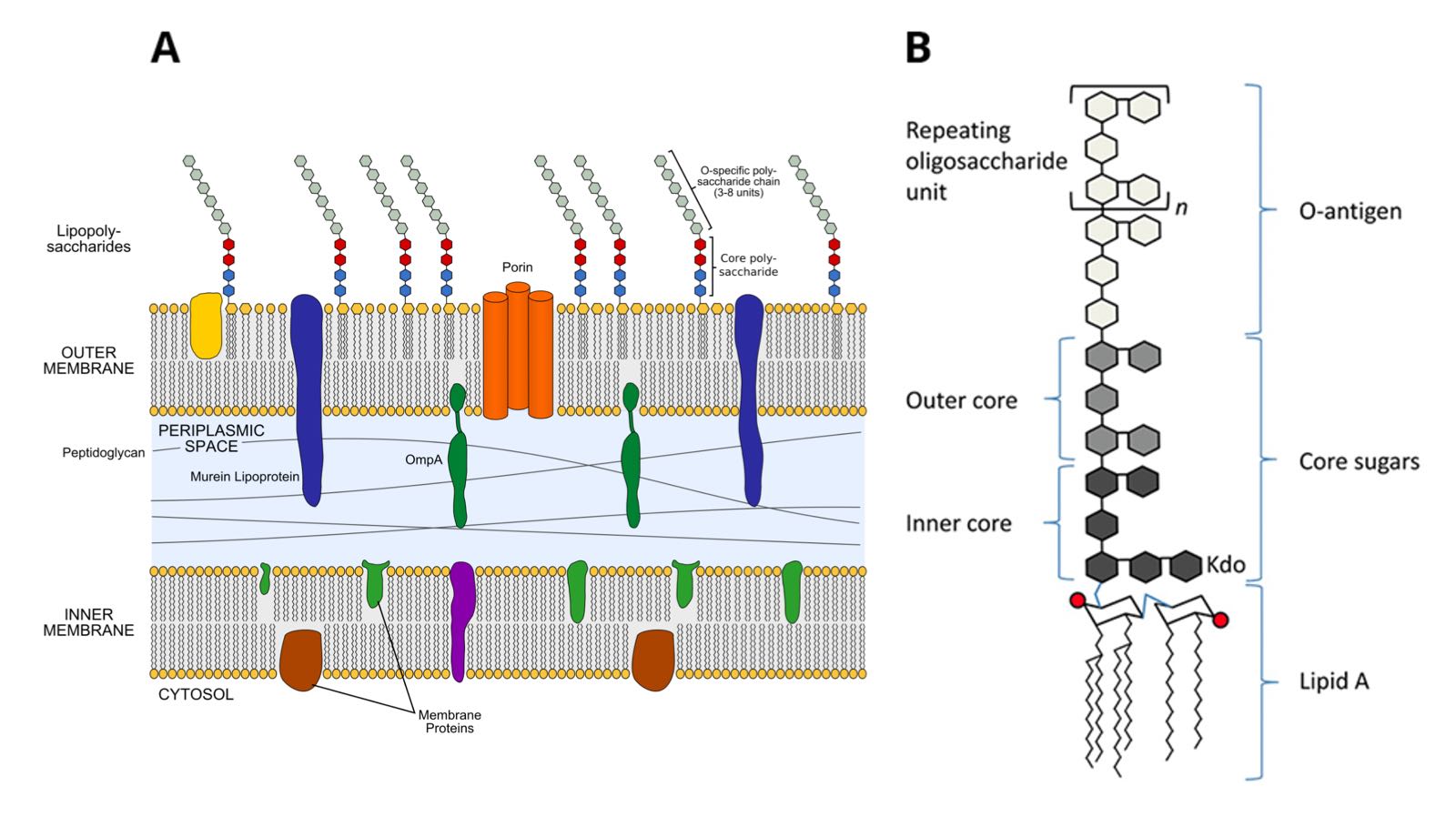
Figure 2: Endotoxin is a major constituent of the outer membrane of gram-negative bacteria A.) Gram-negative cell envelope © Jeff Dahl, CC BY-SA 4.0 via Wikimedia Commons (6) B.) Endotoxin structural schematic, CC BY 3.0 (7)
The PyroSmart NextGen® assay uses recombinant proteins to retain the natural biosensing capability of protein zymogens in the hemolymph of the horseshoe crab Limulus Polyphemus. The recombinant proteins derived from the Limulus Polyphemus genes are formulated in an assay mixture with a chromogenic peptide providing the main constituents of the endotoxin detection assay. The assay is commonly run in a 96-well microplate assay format and progress kinetics are observed using a spectrophotometric plate reader with detection wavelength at 405 nm.
Conducting the assay is an involved process necessitating standard curve preparation, positive product control (PPC) preparation, sample arraying, reagent reconstitution and dispensing all of which are time consuming and tedious.
Automating the liquid handling steps used in standard curve, PPC, and sample preparation, along with sample placement on the plate, increases the efficiency and consistency of these assays while reducing the ergonomic burden on analysts. The Andrew+ Robotic System reduces the analyst-required liquid handling and pipetting steps by over 85% per plate. Many analysts run several plates daily with each assay requiring approximately 150 individual pipetting steps. The OneLab protocol provided below represents a basic procedure for executing the PyroSmart NextGen® assay using one of the most common configurations. Example standard curve, sample, PPC, and recovery data using this protocol are presented in Figure 3 and Figure 4 below.
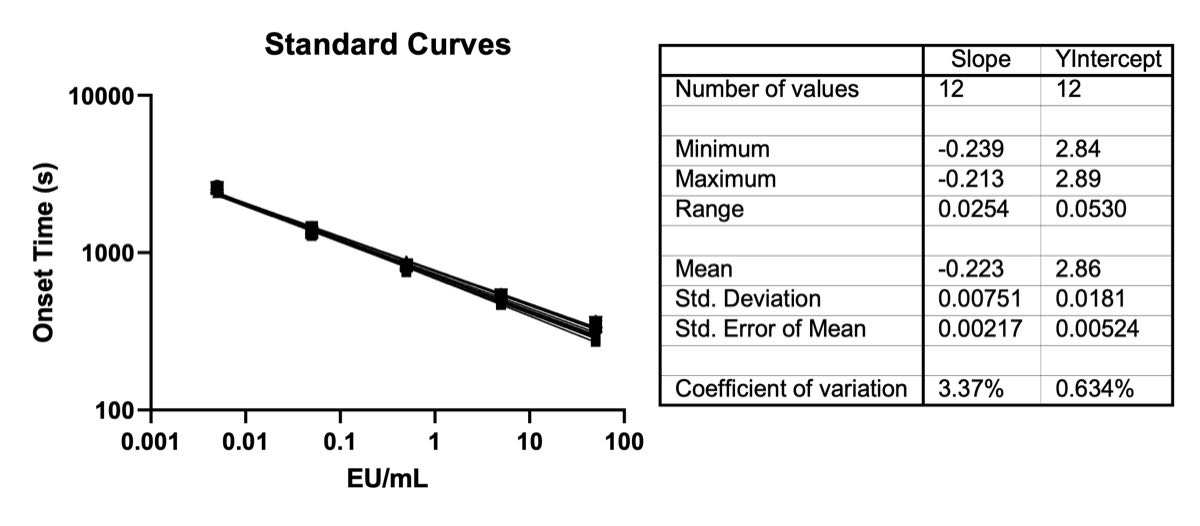
Figure 3: Example Standard Curves for PyroSmart NextGen® executed by Andrew+. Twelve plates of data were collected on at least 4 different days using identical lots of reagents and reference standard endotoxin. A standard curve for each plate was constructed and dispensed from a new set of dilutions for each plate. Linear fits were performed on log-log plots from each plate. The standards used for these curves are 50, 5, 0.5, 0.05, and 0.005 EU/mL. The associated plot has all onset time replicates included (there are a total of 36 points per x-axis value).
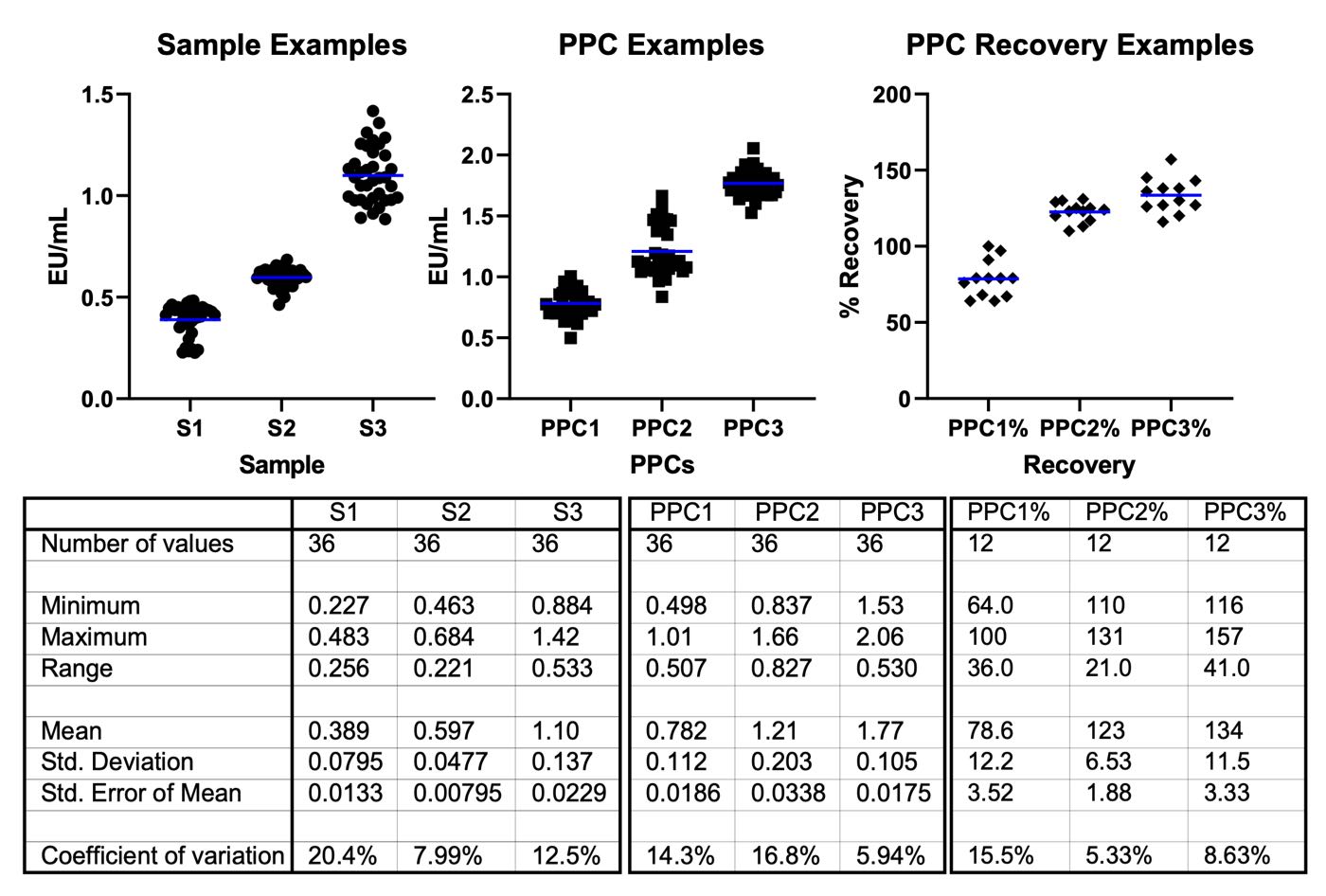
Figure 4: Example Sample PPC and Recovery results for PyroSmart NextGen® executed by Andrew+. The line in the scatter plot indicates the mean value for each set. The results are derived from the same plates as in Figure 3 and EU/mL results calculated from their associated standard curves. Thirty-six data points were collected over 12 plates for each Sample and PPC. PPCs were made by spiking 270 μL of each sample with 30 μL of 5.0 EU/ml standard, targeting a 0.5 EU/mL final concentration. PPC recovery calculated by dividing the spike target concentration (0.5 EU/ml) by the measured concentration of each point, shown as a percentage.
Assay notes
LAL endotoxin assay execution note:
The PyroSmart NextGen® assay can detect minute quantities of endotoxin in down to the ~0.005EU/mL (EU: endotoxin units) range (i.e., sub nanogram (10-9 g/mL). As indicated in the instructions for use (IFU) all materials used for assay execution must be tested for potential interferences. Excellent lab technique and a clean environment are required to execute these assays. To address these requirements, it is suggested that all manipulations be carried out in laminar flow hood or static enclosure.

Figure 5: Andrew+ OneLab Deck set up for the PyroSmart NextGen® Assay Material locations on Andrew+ deck: [1-3] Tips, [3] 96-well microplate, [4] Samples, Standards, PPCs (Positive Product Controls), 1.5 mL conical tubes, [6] LRW, 15 mL centrifuge, 16x90mm glass tubes or ACC tilted vial domino, Note: LRW is LAL reagent water, [7] PyroSmart NextGen reagent, 10 mL reservoir.
Protocol specifications
PyroSmart NextGen® Andrew OneLab Protocol Verified:
- Estimated time of execution: ≈30m
- Hands-on time: 6m
- Tip consumption:
- 6× 10-300 μL tips
- 7× 50-1000 µL tips
- 8× 50-1200 µL tips
Ordering information
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
- Andrew+ Pipetting Robot
- OneLab software
- 2× Tip Insertion System Domino | p/n 186009612
- Microtube Domino | p/n 186009601
- Microplate Domino | p/n 186009600
- 16×90mm Glass Tube Domino | p/n 186010159
- 8-Channel Pipette Reservoir Domino | p/n 186009613
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 1200 μL | p/n 186009615
Additional suggested items
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 5 mL | p/n 186009608
- Tip Insertion System Domino | p/n 186009612
- Tilted Vial Domino | p/n 186010572
- 15mL Conical Centrifuge Tube Domino | p/n 186010087
- Tube Shaker+ with Tilted Vial Adaptor | p/n 186010488
Recommended Consumables
- ACC, Pyroclear® Pyroplate® 96-well microplate | p/n CA961-50
- INTEGRA 10 mL multichannel reservoir | p/n 4332
- Eppendorf 1.5 mL Safe-Lock tube | p/n 0030120086
- ACC, Pyrotube® 16x90mm depyrogenated glass tube | p/n TB16C
References
- Bacterial Endotoxins Test, United States Pharmacopoeia <85>. https://www.usp.org/harmonization-standards/pdg/general-methods/bacterial-endotoxins
- PyroSmart NextGen® Instructions for use https://www.acciusa.com/tools-and-resources/package-insert-sheets
- Lindsay, G. K., P. F. Roslansky, and T. Novitsky. 1989. Single-Step, Chromogenic Limulus Amebocyte Lysate Assay for Endotoxin J. Clinic. Microbiol. 27:947-951. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC267460/
- Stevens, I., Ogura, N., Kelley, M., D’Ordine, R.L., Mizumura, H., Oda, T., Akiyoshi, J. and Jahngen, E.G., 2022. Advanced recombinant cascade reagent PyroSmart NextGen® for bacterial endotoxins test as described in the pharmacopeias. BPB Reports, 5(5), pp.105-114. https://www.researchgate.net/publication/364954840_Advanced_Recombinant_Cascade_Reagent_PyroSmart_NextGenR_for_Bacterial_Endotoxins_Test_as_Described_in_the_Pharmacopeias
- Prior, R.B., 1990. The Limulus amoebocyte lysate test. In Clinical Applications of the Limulus Amoebocyte Lysate Test (p. 27). CRC Press Boca Raton, FL.
- Needham and Trent Nat .Rev. Micro., 2013, Fortifying the barrier: the impact of lipid A remodeling on bacterial pathogenesis, 11:467–4812. https://www.nature.com/articles/nrmicro3047
- Anspach, J. Biochem. Biophys. Methods, 2001, Endotoxin removal by affinity sorbents 49, 665-681. https://pubmed.ncbi.nlm.nih.gov/11694310/
Protocols

Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 