or start from open source methods. Learn more about OneLab softwareUse OneLab
Intracellular Flow Cytometry
This example method provides a freely adjustable framework for measuring the adaptability of the OneLab environment to workflows from different application fields. It helps to understand various nuances of the code-free, universal protocol designer and provides general indications as to the feasibility of a project. Scripts generally require fine adjustment to correct for variables and support specific labware implementation.
Overview
Flow cytometry is widely used in basic and clinical research to identify, quantify, and sort distinct cell phenotypes within a heterogeneous population of cells. This application, known as immunophenotyping, is commonly used to detect specific immune cell types in a complex population using fluorophore-conjugated antibodies directed against specific cell surface markers. Flow cytometry also enables the analysis of intracellular processes, such as cell signaling, using antibodies to detect target signaling proteins and their induced post-translational modifications (e.g. phosphorylation) as well as activated downstream transcription factors and regulatory proteins.
The staining of intracellular target molecules involves the fixation of cells in suspension and their subsequent permeabilization before the addition of the detection antibody. The fixation/permeabilization treatment immobilize and preserve target proteins in a transient state and allow the antibody to pass through the plasma membrane into the cell interior while maintaining the morphological characteristics used to sort the cells. Commonly used detergents to permeabilize cell membranes include saponin, Triton™ X-100, or Tween®20.
Immunofluorescence Staining
Two immunofluorescence techniques can be applied for staining (Figure 1). The indirect method uses a non-fluorescent target-specific primary antibody, which is probed by a fluorophore-conjugated secondary antibody. The second approach utilizes a single fluorophore-conjugated detection antibody specifically directed against the biomolecule of interest.
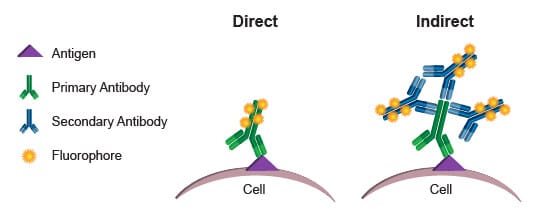
Figure 1: Direct versus indirect immunostaining for flow cytometry (1).
Intracellular Staining Protocol for Flow Cytometry
This protocol is designed for the staining of cells for flow cytometry measurement of intracellular proteins. Cells are firstly fixed and then permeabilized, allowing direct staining of intracellular molecular markers using a fluorophore-conjugated primary antibody (Figure 2). The number of cells, containing or expressing the target biomolecule, is counted on a flow cytometer, either with or without sorting depending on the actual application. It is recommended that experimental conditions, such as antibody concentration, incubation time, and temperature, be optimized for each flow cytometry experiment.
Watch this brief introduction to flow cytometry from Cell Signaling Technology.
(1) Cell Signaling Technology. “Overview of Flow Cytometry.”
Protocols

Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 