or start from open source methods. Learn more about OneLab softwareUse OneLab
Intact Mass and Cell Culture Media Workflows

This advanced method offers an end-to-end solution developed around an advanced application, a specialized kit, or an analytical system. It delivers complete functional, ready-to-use protocols that are qualitatively and quantitatively assessed for consistency, executability, and repeatability, usually without needing to make any changes. It operates per batch size and is scalable to accommodate changing requirements.
Overview
Waters Bioprocess Walk-Up Solutions utilizes OneLab as a single-access platform for controlling sample preparation and LC-MS data acquisition and reporting
Enhancements enabling plate sealing and direct to LC-MS plate handling and triggering have been introduced.
Several new sample prep protocols/capabilities are now available, depending on the assay needs; simple dilute and shoot, plate-based filtration to clarify samples, and Protein A magnetic bead plate-based clean up.
Cell culture or microbial-based inoculation for protein production is a time-consuming process, typically lasting about two weeks. It is increasingly desirable to routinely monitor critical process and product attributes such as changes in nutrient profiles and high-level glycoform information for the drug substance. Monitoring feed and metabolite components will aid in designing or optimizing feed strategies for nutrient replenishment; detect and quantify the formation of toxic metabolites; and can be used to elucidate what reactor conditions are conducive to optimal cell growth and bioproduction. Higher monitoring frequency of the protein formation in the process enables the determination of the most favorable cell culture duration; yield optimization; and most importantly ensures that the drug substance quality is within the specification criteria.
Moving these assays and technologies in the hands of bioprocessing groups facilitates faster access to more in-depth information for the process engineers.
These protocols, as part of a walk-up solution, enable robust, routine LC-MS analysis in bioprocess laboratories. A single interface based on OneLab software provides a seamless connection between sample input, automated sample preparation, and LC-MS data acquisition and analysis (Figure 1).
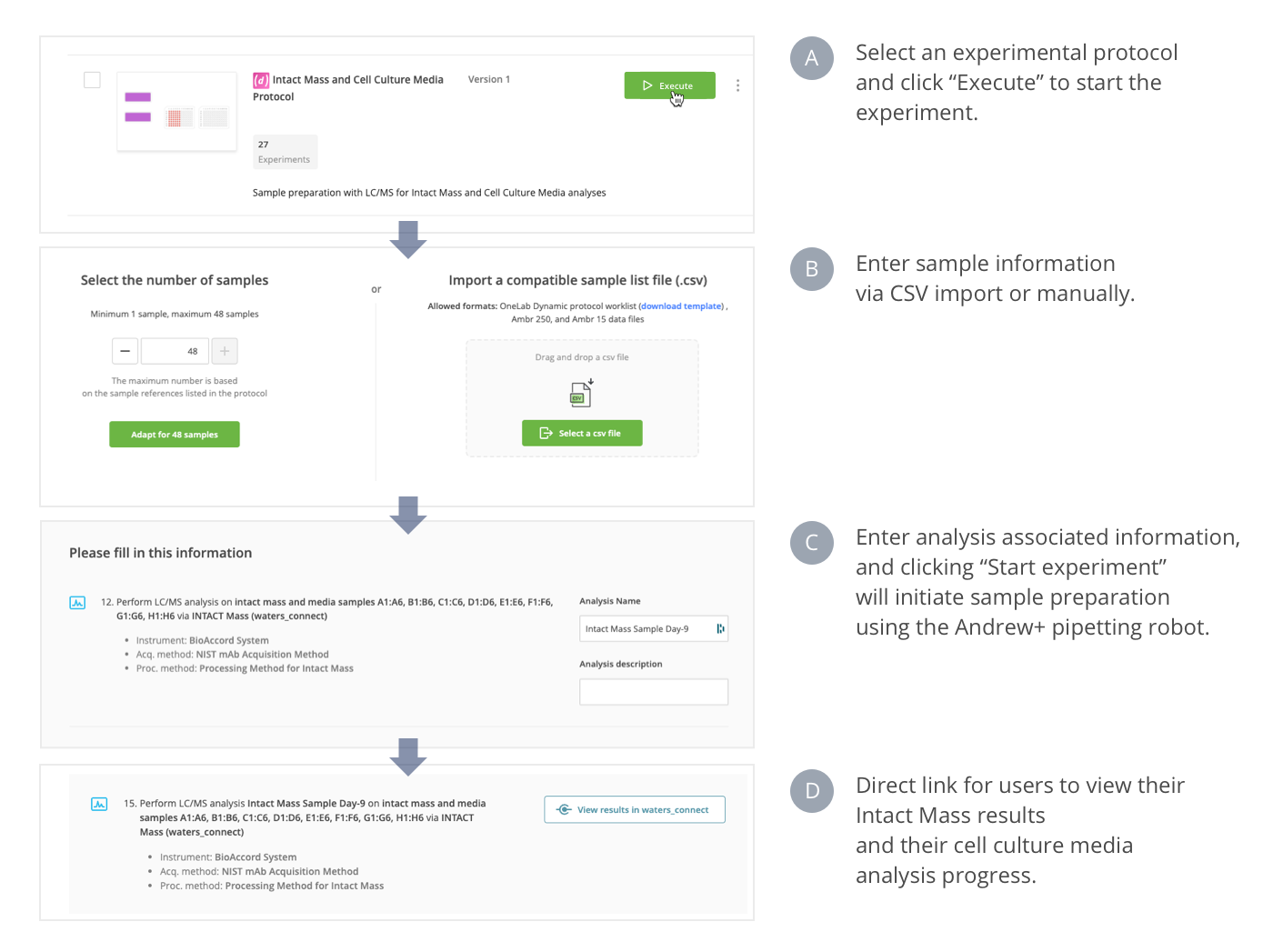
Figure 1: Top-level flow chart for the walk-up solution using single access in the OneLab software platform
In addition, users of Sartorius Ambr® bioreactors can utilize the data interface to automatically upload all sample information and return their results back to the Ambr software for further interrogation. The interface, combined with predeveloped analytical workflows for key product and process quality attributes, are designed to allow bioengineers to perform assays generating high-quality data with minimal training in LC and MS technologies.
Experimental
The following protocols demonstrate the capabilities of the current Bioprocess Walk-Up Solutions and Andrew Alliance Pipetting Robot updates including the Plate Sealer, the LC-MS Portal as well as existing capabilities such as the Magnet+ and Extraction+ smart devices for Bioprocess applications. Combining protocols to design new custom workflows may be possible, depending on throughput and needs.
Protocol 1 – Intact Mass and Cell Culture Media Analysis with Sealing
The protocol will process up to 48 clarified media samples in a 350 µL 96-well plate, seal the plate of samples and transfer the samples to the LC-MS via the portal. The samples will be automatically diluted using a diluent that is the same as the starting mobile phase of the LC-MS assays to be run. At the end of the protocol, a single 350 µL 96-well plate is produced that contains ~180 µL samples that have been diluted to 1/20 suitable for intact mass analysis on the left side of the plate, and also 200 µL samples diluted to 1/400 on the right side of the plate that are appropriate for cell culture media analysis. Once the plate is prepared, the samples are transferred to the BioAccord and acquisitions may be run. This sample preparation is appropriate for Intact Mass analysis (light chain and mAb analysis) and Cell Culture Media analysis workflows described below. Note: Protocol 1 requires samples which have been clarified during or immediately post-harvest via 0.1/0.2 µm based filtration and/or centrifugation.
Protocol 2 – Intact Mass and Cell Culture Media Analysis with Clarification
This protocol will process up to 24 media samples that require clarification (or reclarification). Note: Achieving quality and representative results requires harvested samples to be processed (cell and cell debris removed) as soon as possible, and is generally recommended to be performed in less than 1 hour to ensure consistent, accurate results. This procedure also prepares two sets of samples with final dilution at 1/20 and 1/400 suitable for intact mass and cell culture media analysis. The clarification protocol may also be combined with other LC-MS based sample analysis that requires cell free harvested samples or in conjunction with additional sample preparation steps.
Protocol 3 – Protein A Magnetic Bead Purification
This protocol is designed to process up to 8 samples that require a magnetic bead based sample preparation. This protocol was designed to take clarified samples and perform affinity cleanup. Several brands of Protein A magnetic beads are available depending on application need, the base method was performed using Magne® Protein A Beads (Promega). This protocol is appropriate to be used before an Intact Mass analysis, SEC based analysis or prior to additional sample preparation (for example subunit or peptide level digestion) approaches.
Intact Mass Analysis
Once the plate has been transferred to the BioAccord, the 1/20 diluted samples will be automatically run and processed by waters_connect using the INTACT Mass application (Figure 2). For more details on application features and functionality, please refer to the application note (Reference 2).
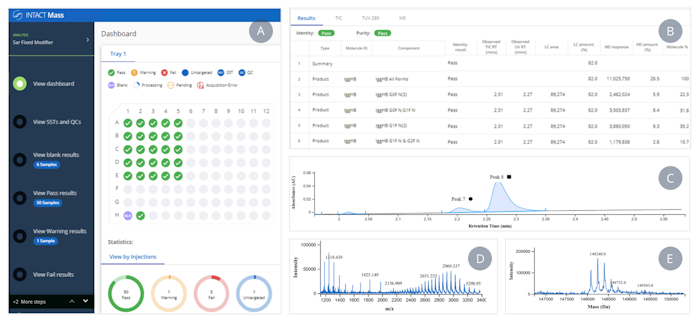
Figure 2: Intact Mass Dashboard. Results are displayed in a simple layout containing: a simple data review workflow and a plate map (A); major glycoforms reported per sample (B); and optionally access to chromatographic integrations and spectral data (C, D, E) for each sample.
Cell Culture Media Analysis
Using the same plate as for the intact mass analysis, the 1/400 dilution samples are ready to be run and then processed using the UNIFI application. Samples will be screened against a comprehensive (built-in) library (Figure 3). For more details on application features and functionality, please refer to application note (Reference 3).
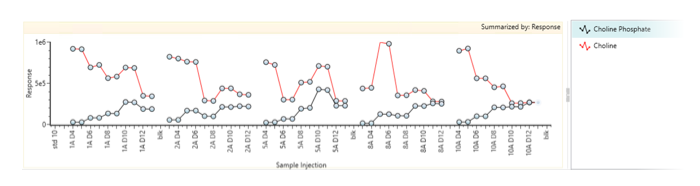
Figure 3: Example of overlaid trending plots (of choline and choline phosphate) across 5 bioreactors using the Cell Culture Media workflow.
These protocols provide several options and workflows for automating sample preparation using the Andrew+™ Pipetting Robot and initiate LC-MS data acquisition for intact protein, and cell culture nutrient and metabolites monitoring in spent media. The combination of BioAccord LC-MS system and Andrew+ Pipetting robot provides the capability to process samples from bioreactor systems and process development teams quickly and easily to provide high-quality results with minimal user interaction.
Highlights and capabilities include:
- A single access and easy-to-use interface in OneLab, with predeveloped workflows, that will initiate sample preparation and subsequent data acquisition in a walk-up manner.
- Automated sample preparation and sample information transfer to maximize productivity and minimize human error.
- Ability to utilize the direct to portal injection mode on the LC system, enabling walk away sample processing, injection and processing of samples.
- Acquire both intact protein and cell culture media data back-to-back based on column switching using a two-column compartment column manager.
- Seamless collection of intact protein glycoprofiling and cell culture media composition will aid process monitoring and understanding.
- The compact and user-friendly BioAccord LC-MS System produced excellent data quality to help support the development of more robust processes.
In conclusion, Waters Bioprocess Walk-Up Solutions will enable process engineers to easily and routinely obtain high-quality data to aid in process monitoring and optimization.
Assay notes
Protocol 1 – Intact Mass and Cell Culture Media Analysis with Sealing
As part of the Bioprocess Walk-Up Solution, this protocol:
- Manages sample information coming from bioreactors and passes the metadata through to the analytical system, removing transcription errors
- Executes automated sample preparation using the Andrew+ Pipetting Robot
- Dynamically scales sample preparation to the number of samples provided
- Automatically creates a LC-MS sample run list
- Initiates the BioAccord LC-MS data acquisition for glycoform determination using the INTACT Mass application
- Optionally sends back intact protein (relative glycoform levels) back to be visualized as variables in Ambr® systems via a data interface
- Creates samples to be run for cell culture media analysis
Clarified bioreactor samples (centrifuged and 0.2 µM filtered samples) are prepared for analysis via multiple sample dilution steps, creating a plate that contains samples ready for intact mass analysis (the determination of major relative glycoforms) and samples ready for spent cell culture media analysis (the determination of levels of feed components and metabolites). Additional protocols for plate based clarification and protein A based cleanup are also provided and may be integrated into custom workflows.
User must ensure that samples are centrifuged and/or filtered prior to injection of samples, the filtration protocol enables one way to automate this practice.
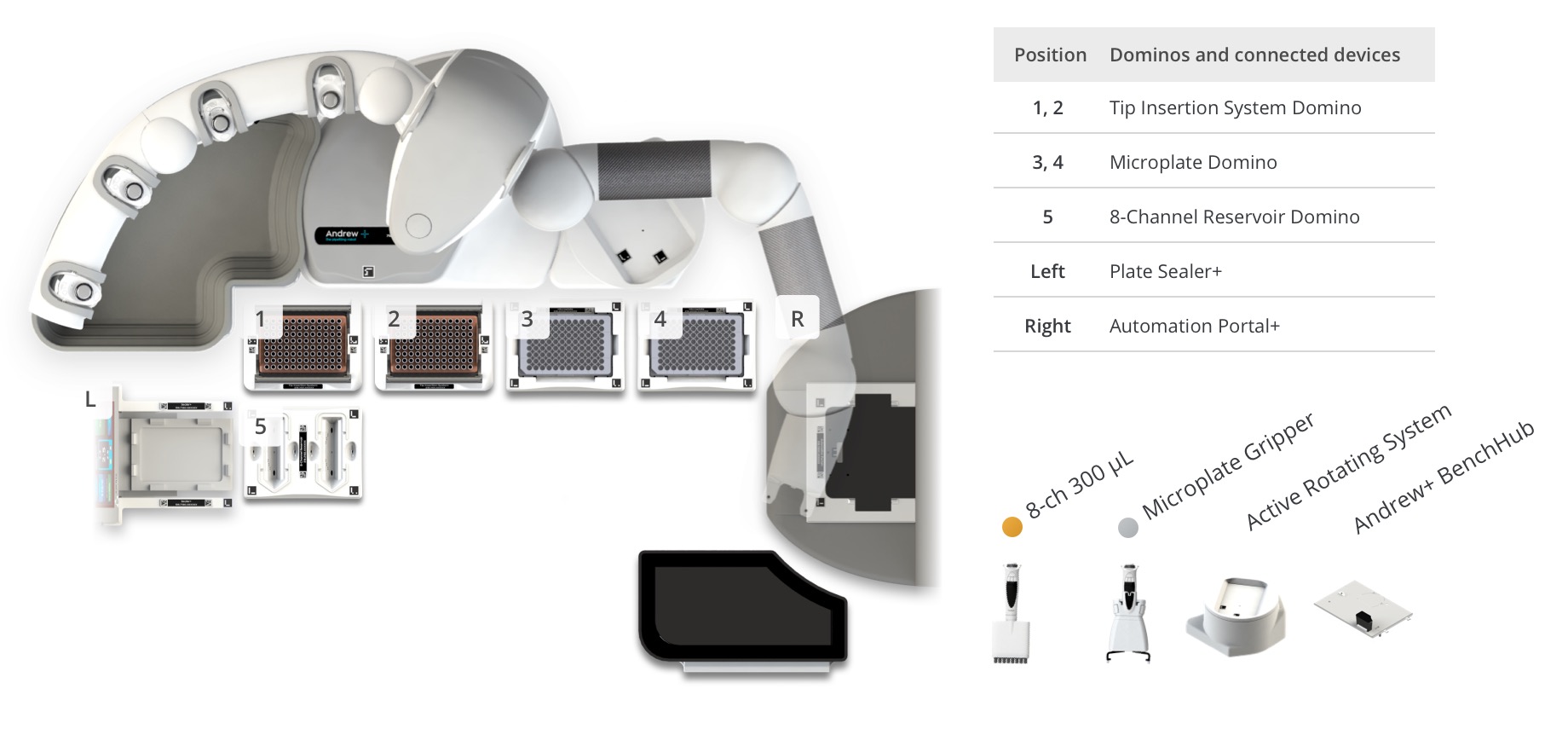
Figure 4: Andrew+ OneLab deck setup for the Intact Mass and Cell Culture Media workflows with plate heat sealing and automated plate loading (48 samples). Samples and reagents locations on the Andrew+ deck: [1-2] Tips, [3] 96 well microplate will be transferred in the BioAccord, [4] Pre-centrifuged and/or pre-filtered samples, [5] 8-channel reservoir contains 0.1% Formic acid reagent, [L - Left] Plate sealer+, [R - Right] Automation Portal
Protocol 2 – Intact Mass and Cell Culture Media Analysis with Clarification
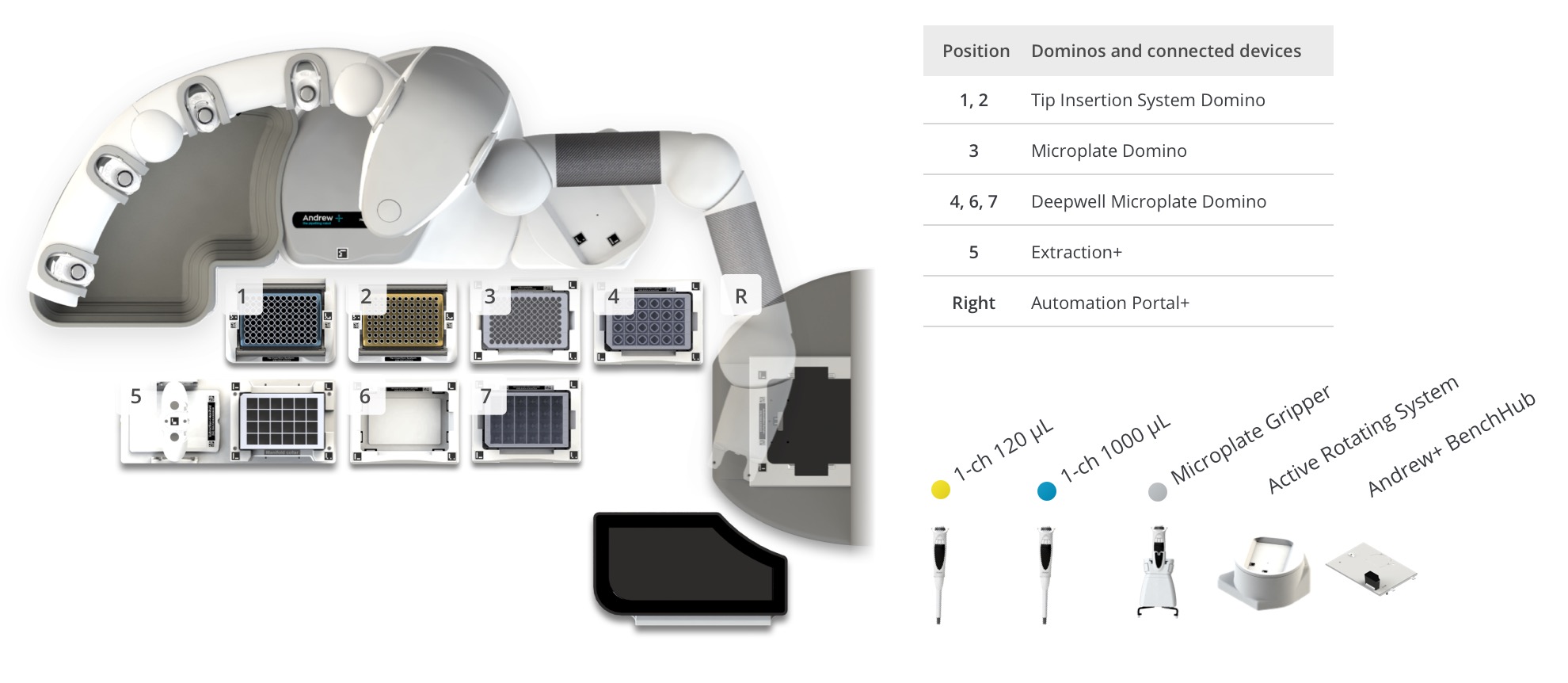
Figure 5: Andrew+ OneLab deck setup for the Intact Mass and Cell Culture Media workflows with clarification and automated plate loading (24 samples). Samples and reagents locations on the Andrew+ deck: [1-2] Tips, [3] 96 well microplate will be transferred in the BioAccord, [4] Samples, [5] Extraction+ vacuum manifold with clarification plate and collection plate, [6] Empty for collection plate reception, [7] 6-column reagent reservoir contains 0.1% Formic acid reagent, [R - Right] Automation Portal+
Protocol 3 – Protein A Magnetic Bead Purification
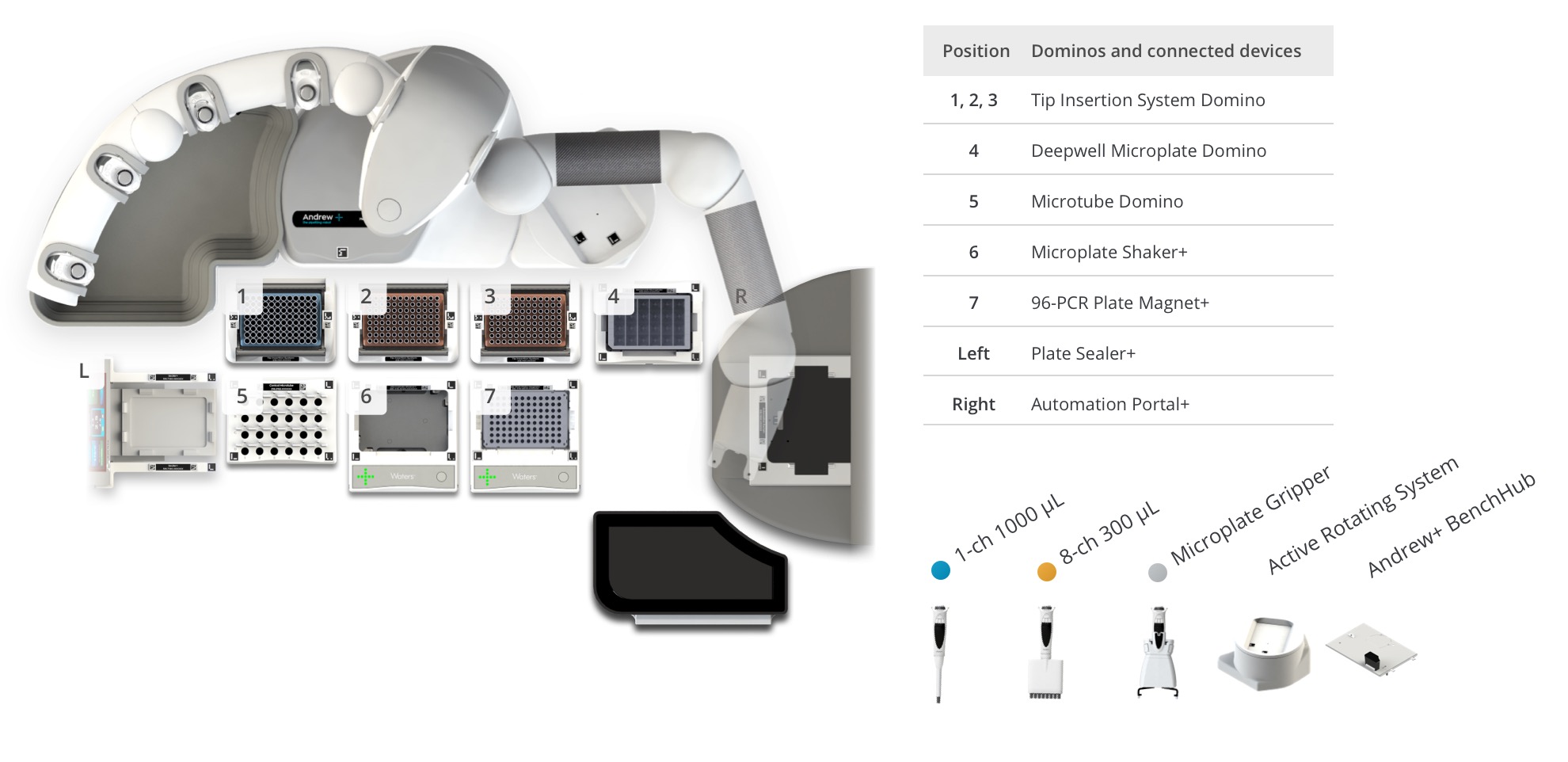
Figure 6: Andrew+ OneLab deck setup for the Intact Mass workflows with ProA Mag bead purification and automated plate loading (8 samples). Samples and reagents locations on the Andrew+ deck: [1-3] Tips, [4] 6-column reagent reservoir contains reagents, [5] Magnetic beads slurry, [6] Microplate Shaker+, [7] 96-PCR Plate Magnet+ with samples at start, plate will be transferred in the BioAccord [L - Left] Plate sealer+, [R - Right] Automation Portal+
Protocol specifications:
Intact Mass and CCM Protocol with Clarification:
- Estimated time of execution: 1 h 2 min
- Hands-on time: 0 s
- Tip consumption:
- 48× 5-120 μL tips
- 26× 50-1000 μL tips
Intact Mass and CCM Protocol with Sealing:
- Estimated time of execution: 14 min
- Hands-on time: 0 s
- Tip consumption:
- 104× 10-300 μL tips
ProA Mag bead purification for 8 media samples in PCR plate:
- Estimated time of execution: 40 min
- Hands-on time: 0 s
- Tip consumption:
- 160× 10-300 μL tips
- 8× 50-1000 μL tips
Ordering information
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
- Andrew+ Pipetting Robot
- Waters BioAccord System
- OneLab software Enterprise deployment
- OneLab Intact Mass Integration module | p/n 716012207
- 3× Tip Insertion System Domino | p/n 186009612
- 2× Microplate Domino | p/n 186009600
- 8-Channel Pipette Reservoir Domino | p/n 186009613
- 3× Deepwell Microplate Domino | p/n 186009597
- Plate Sealer+ w/ Bench (Automated Plate Sealer, Plate Sealer integration kit, Plate Sealer bench | p/n 176005829
- Acquity Automation Portal | p/n 725000672
- Automation Portal Base Kit (Automation Portal integration kit, Active Rotating System (ARS) | p/n 176005830
- Andrew+ BenchHub | p/n 186011278
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 120 μL | p/n 186009765
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Microplate gripper | p/n 186009776
- 2× Pipette Adaptor Single Channel | p/n 186009590
- 1× Pipette Adaptor Multi-Channel | p/n 186009591
For clarification workflow
For ProA Mag bead purification workflow
Consumables
- Waters 350 µL 96-round well collection plate (Pk100) | p/n 186002643
- Polypropylene Cap Mat Round Well for 96-well Plate (Pk50) | p/n 186002483
- INTEGRA 10 mL multichannel reservoir | p/n 4332
- Agilent 6-column reagent reservoir | p/n 201284-100
- Sartorius, Optifit Tips, 0.5-200 µL | p/n 700013295
- Sartorius, Optifit Tips, 5-350 μL | p/n 700013297
- Sartorius, Optifit Tips, 10-1000 μL | p/n 700013298
- Azenta Clear Heat Seal film | 4ti-0540
- Cytiva AcroPrep cell clarification and sterile filtration Plate, 24 well, 7 mL | 97026
- Promega Magne® Protein A Beads | G8781
- Eppendorf twin.tec PCR Plate 96, skirted, 200 μL/well | 951020443
Columns
- ACQUITY Premier HSS T3 Column 1.8 µm, 2.1 x 150mm, 1/pk | p/n 186009469
- ACQUITY Premier Protein BEH C4 300 Å 1.7 µm, 2.1 x 50 mm, 1/pk | p/n 186010326
Certificates
- BioAccord System Install Certificate | p/n 741000510
- Andrew+ Robot System Installation Cert. | p/n 741000516
- Remote Andrew+Robot Sys IMPL Certificate | p/n 741000521
References
- Waters Application Note 720008062EN - Simplifying Bioreactor In-Process Monitoring With Waters Bioprocess Walk-Up Solutions (https://www.waters.com/nextgen/en/library/application-notes/2023/simplifying-bioreactor-in-process-monitoring-with-the-bioprocess-walk-up-solution.html).
- Waters Application Note 720007547 - INTACT Mass™ - a Versatile waters_connect™ Application for Rapid Mass Confirmation and Purity Assessment of Biotherapeutics (https://www.waters.com/nextgen/en/library/application-notes/2022/intact-mass-a-versatile-waters-connect-application-for-rapid-mass-confirmation-and-purity-assessment-of-biotherapeutics.html).
- Waters Application Note 720007359 - Monitoring Nutrients and Metabolites in Spent Cell Culture Media for Bioprocess Development Using the BioAccord LC-MS System With ACQUITY Premier (https://www.waters.com/nextgen/en/library/application-notes/2021/monitoring-nutrients-and-metabolites-in-spent-cell-culture-media-for-bioprocess-development-using-the-bioaccord-lc-ms-system-with-acquity-premier.html).
Protocols


Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 