or start from open source methods. Learn more about OneLab softwareUse OneLab
Indirect Sandwich ELISA

This example method provides a freely adjustable framework for measuring the adaptability of the OneLab environment to workflows from different application fields. It helps to understand various nuances of the code-free, universal protocol designer and provides general indications as to the feasibility of a project. Scripts generally require fine adjustment to correct for variables and support specific labware implementation.
Overview
ELISA, a Gold Standard for Diagnosis, Detection, and Validation
Enzyme-Linked Immunosorbent Assay, typically known as ELISA, is a qualitative and quantitative immunoassay that uses antibodies as probes to specifically detect and quantify molecules of interest, such as proteins, peptides, antibodies, and hormones. It relies both on the ability of immunoglobulins to bind specifically to a target antigen and a detection system whose measurable signal directly reports on the presence and quantity of the antigen. Besides being a powerful research tool for antibody development and screening, the ELISA technique is broadly used in clinical diagnostics as a biomarker detection assay in various areas including cancer, diabetes, cardiovascular disease, viral infection, and inflammation. It is also useful for toxicology testing and detecting allergens in the food industry.
Although several types of ELISA have been developed, they all comprise four steps: coating, blocking, detection, and signal measurement. The experimental setup of ELISA will then vary depending on the biological question, the antigen or analyte to be investigated, and the detection strategy. A typical ELISA test begins with immobilizing the target antigen on a solid surface, generally the polystyrene surface of a multi-well microplate, either by passive adsorption in alkaline conditions (direct coating) or indirectly (indirect coating) using antigen-specific antibodies adsorbed onto the plate surface. Following incubation with the antigen sample, all unsaturated surface-binding sites are covered with a blocking agent to prevent the non-specific binding of proteins to the plate, thereby maximizing the signal-to-noise ratio. The detection of the bound antigen involves two different methods: direct and indirect. The direct detection method uses an enzyme or fluorophore-conjugated antibody that directly recognizes the antigen, while the indirect method consists of probing the antigen-specific primary antibody with a labeled secondary antibody. A common adaptation of indirect detection involves the use of a streptavidin/avidin-enzyme conjugate to bind the biotinylated detection antibody, resulting in signal amplification. Washing between steps is crucial to ensure that non-specific (low affinity) binding events are removed, thus eliminating the background noise. Finally, when using an enzyme, the appropriate substrate is added to trigger the enzymatic reaction and consequently produce a measurable signal, which is either a change of color, fluorescence or luminescence. The intensity of the signal is proportional to the amount of antigen bound to the antibodies and can, therefore, be used as a readout to determine the antigen concentration in the sample by comparing it to a standard curve for the equivalent antigen (generally a purified recombinant protein). The most commonly used enzymes are horseradish peroxidase (HRP) and alkaline phosphatase (AP). The choice of substrate will depend on the instrumentation available for signal measurement but also on the degree of sensitivity required for the assay. Using chemiluminescence, ELISA is potentially able to detect antigens in the sub-picogram per well range, while colorimetric and chemi-fluorescence detections are able to measure low-to-mid picogram levels of antigen per well. When using a fluorophore, no enzyme or substrate is required. The fluorescent moiety is covalently attached to the secondary antibody or in some cases to the detection antibody or the streptavidin/avidin molecule. The signal is measured using a fluorometer.
Sandwich or Capture ELISA
In a Sandwich ELISA, the target antigen is recognized by two specific antibodies, thus forming a sandwich-like complex. In the simplest form of sandwich ELISA, the first antibody, named capture or coating antibody, is adsorbed onto the well surface and recognizes specifically the target antigen (Figure 1). The second antibody binds to the captured antigen and is called the detection antibody. These two antibodies form a matched pair that essentially undergoes development and validation in order to ensure their ability to work in combination and bind to two distinct, non-overlapping epitopes on the antigen molecule. When developing a matched pair, it is crucial to use two antibodies that have been raised in different host species to prevent cross-reactivity between the coating and detection antibodies. Moreover, a set of monoclonal and polyclonal antibodies can be used, where the monoclonal acts as a coating antibody (for high-specificity binding) and the polyclonal for detecting the antigen. The detection antibody can be either labeled with an enzyme for direct detection (Figure 1) or probed by an enzyme-conjugated secondary antibody specifically directed against the detection antibody (indirect detection). Alternatively, a biotinylated detection antibody can be introduced, which then enables the use of streptavidin-enzyme conjugate (Figure 1). Sandwich ELISA is a highly specific assay because only the target antigen/analyte is captured and detected with the matched antibody pair. This makes it suitable to analyze complex protein samples since the antigen/analyte does not require purification prior to measurement. Sandwich ELISA often requires more optimization than traditional (direct and indirect) ELISAs but delivers higher sensitivity and signal-to-noise ratio (minimal background signal).
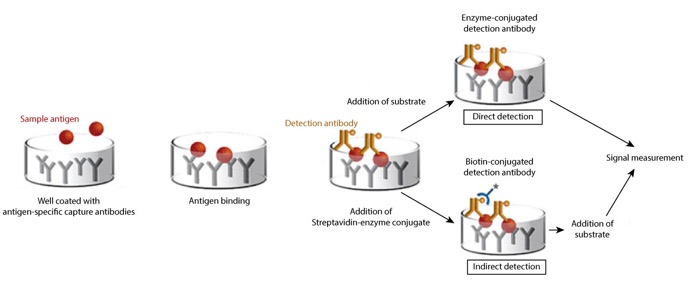
Figure 1: A schematic illustration of Sandwich ELISA highlighting two different detection systems; Direct using enzyme-conjugated detection antibody and indirect using the Streptavidin-enzyme conjugate to probe the biotinylated detection antibody.
General Sandwich ELISA Protocol
This procedure represents a general sandwich ELISA involving a biotinylated detection antibody and a streptavidin-HRP conjugate for indirect colorimetric detection using the TMB (tetramethyl benzene) substrate. Protocols can be easily modified and adapted depending on your samples and the working concentrations of your matched antibody pair and enzyme conjugate.
The procedure comprises 4 steps:
- Protein Standard Preparation,
- Plate coating with capture antibody and plate blocking prior to the addition of standards and samples,
- Incubation with biotinylated detection antibody and enzyme conjugate,
- Addition of substrate and colorimetric detection.
The “Protein Standard Preparation” step consists in preparing a serial dilution of the antigen/analyte of interest in order to generate a standard curve. The Concentration of the antigen/analyte in samples is then extrapolated from the standard curve within the appropriate dynamic range. To obtain accurate concentration measurement, the standard curve should display low variation between replicates and good linearity.
Recommendations and Good Practices
To achieve high accuracy and performance in ELISA, it is crucial to:
- Ensure sample quality and integrity to obtain consistent results,
- Adhere to incubation parameters (time, temperature, light exposure) for optimal antigen binding, detection, and signal intensity,
- Perform proper and thorough blocking and washing to thwart any non-specific interference and eliminate background noise,
- Use suitable multi-well plates, such as polystyrene (PS) or polyvinyl chloride (PVC) 96-well flat-bottom microplates,
- Do not allow the plate to dry out during the assay.
Automation of Indirect Sandwich ELISA Using the Andrew+ Pipetting Robot
The automation of the ELISA procedure is achieved using 4 protocols:
- Protein Standard Preparation with Pipette+ (Figure 2),
- Plate Coating, Blocking, and Sample-Standard Addition using Andrew+ (Figure 3),
- Incubation with Detection Antibody and Enzyme Conjugate using Andrew (Figure 4),
- Substrate Addition and Colorimetric Detection using Andrew+ (Figure 5).
∎ Protocol 1 | ELISA – Protein Standard Preparation - Pipette+
∎ Protocol 2 | ELISA – Plate Coating, Blocking, and Sample-Standard Addition - Andrew+

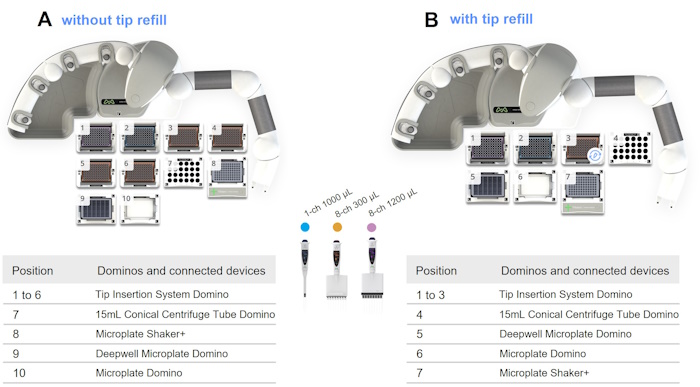
∎ Protocol 3 | ELISA – Incubation with Detection Antibody and Enzyme Conjugate - Andrew+
∎ Protocol 4 | ELISA – Substrate Addition and Colorimetric Detection - Andrew+

Protocol Specifications
▶ ELISA – Plate Coating, Blocking, and Sample-Standard Addition - Andrew+
- Estimated time of execution | 3h 48min 28sec
- Tip consumption | 56x 5-350 μL tips, 1x 10-1000 μL tips, and 32x 50-1200 μL tips
▶ ELISA – Incubation with Detection Antibody and Enzyme Conjugate - Andrew+
- Estimated time of execution | 3h 40min 12sec
- Tip consumption | 352x 5-350 μL tips, 2x 10-1000 μL tips, and 96x 50-1200 μL tips
▶ ELISA – Substrate Addition and Colorimetric Detection - Andrew+
- Estimated time of execution | 22min 16sec
- Tip consumption | 8x 5-350 μL tips, 1x 10-1000 μL tips, and 8x 50-1200 μL tips
ORDERING INFORMATION
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
➤ ELISA – Protein Standard Preparation - Pipette+
- Pipette+ Stand | p/n 176005128
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Sartorius, Optifit Tips, 5-350 μL (x3) | p/n 700013297
- Sartorius Optifit Tips, 10-1000 μL (x3) | p/n 700013298
➤ ELISA – Plate Coating, Blocking, and Sample-Standard Addition - Andrew+ w/ Microplate Shaker+
- Microtube Domino | p/n 186009601
- Microplate Domino | p/n 186009600
- 15mL Conical Centrifuge Tube Domino | p/n 186010087
- Deepwell Microplate Domino | p/n 186009597
- 3x Tip Insertion System Domino | p/n 186009612
- Microplate Shaker+ | p/n 176004577
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 1200 mL | p/n 186009615
- Sartorius, Optifit Tips, 5-350 μL (x56) | p/n 700013297
- Sartorius Optifit Tips, 10-1000 μL (x1) | p/n 700013298
- Sartorius Optifit Tips, 50-1200 μL (x32) | p/n 700013300
➤ ELISA – Incubation with Detection Antibody and Enzyme Conjugate - Andrew+ w/ Microplate Shaker+ (without tip refill)
- Microplate Domino | p/n 186009600
- 15mL Conical Centrifuge Tube Domino | p/n 186010087
- Deepwell Microplate Domino | p/n 186009597
- 6x Tip Insertion System Domino | p/n 186009612
- Microplate Shaker+ | p/n 176004577
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 1200 mL | p/n 186009615
- Sartorius, Optifit Tips, 5-350 μL (x352) | p/n 700013297
- Sartorius Optifit Tips, 10-1000 μL (x2) | p/n 700013298
- Sartorius Optifit Tips, 50-1200 μL (x96) | p/n 700013300
➤ ELISA – Incubation with Detection Antibody and Enzyme Conjugate - Andrew+ w/ Microplate Shaker+ (with tip refill)
- Microplate Domino | p/n 186009600
- 15mL Conical Centrifuge Tube Domino | p/n 186010087
- Deepwell Microplate Domino | p/n 186009597
- 3x Tip Insertion System Domino | p/n 186009612
- Microplate Shaker+ | p/n 176004577
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 1200 mL | p/n 186009615
- Sartorius, Optifit Tips, 5-350 μL (x352) | p/n 700013297
- Sartorius Optifit Tips, 10-1000 μL (x2) | p/n 700013298
- Sartorius Optifit Tips, 50-1200 μL (x96) | p/n 700013300
➤ ELISA – Substrate Addition and Colorimetric Detection - Andrew+
- 2x Microplate Domino | p/n 186009600
- 15mL Conical Centrifuge Tube Domino | p/n 186010087
- Deepwell Microplate Domino | p/n 186009597
- 3x Tip Insertion System Domino | p/n 186009612
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 1200 mL | p/n 186009615
- Sartorius, Optifit Tips, 5-350 μL (x8) | p/n 700013297
- Sartorius Optifit Tips, 10-1000 μL (x1) | p/n 700013298
- Sartorius Optifit Tips, 50-1200 μL (x8) | p/n 700013300
Find a complete overview of ELISA
Learn all about ELISA development and optimization
Download ELISA technical guide including General Sandwich ELISA Protocol
Protocols


Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 