or start from open source methods. Learn more about OneLab softwareUse OneLab
GlycoWorks® Reducing Protocol
This basic method provides the core methodology for translating a workflow into OneLab-executable script(s) as an attempt to fully or semi-automate a specific procedure. It demonstrates the benefits of automation and highlights OneLab capabilities and best practices to promote solution adoption, helping transition from manual to a more automated approach. It can be used alone or serves as a building block for a more complex workflow and is easily adaptable to users' requirements.
Overview
N-Glycan Analysis is Challenging, yet Essential for Routine Analysis
Many therapeutic glycoproteins are composed of N-glycans that play critical roles in the folding, conformation, and stability of the proteins. Unlike a chemical synthesis, the biosynthesis of N-glycans is a concerted effort involving multiple enzymes at various stages and consequently, yields several diverse structures of N-glycans – high-mannose, hybrid, and complex. Therefore, the analysis of glycans is filled with significant technical challenges.
Highly sensitive to manufacturing conditions, glycans also affect the efficacy and clinical safety of biotherapeutics. Therefore, drug developers and manufacturers have to monitor, characterize, and identify the glycans released from glycoproteins to ensure the quality of biotherapeutics. In addition, N-glycosylated proteins are analyzed as biomarkers for several diseases such as testicular cancer and ectopic pregnancy. These glycoproteins associated with diseases are increasingly becoming more complex in their structures and heterogeneity. One approach to facilitate the release of the N-glycans is to reduce the disulfide bridges in the glycoprotein to help unfold the glycoprotein, exposing not easily accessible N-glycans for release with PNGase F. For instance, human chorionic gonadotropin (hCG), known for its role in ectopic pregnancy, mainly consists of complex-type N-glycans and multiple disulfide bridges.
Initially, for disulfide-rich glycoproteins, the GlycoWorks procedures outlined the use of Tris(2-carboxyethyl)phosphine (TCEP) as a reductant. While the TCEP reduction is compatible with the HILIC separation of RFMS-labeled glycans, the by-products of the TCEP reduction have been shown to interfere with the reversed-phase (RP) separation of RFMS-labeled glycans. As a result, a procedure has been developed using Dithiothreitol (DTT) as a reductant that does not cause significant interferences for N-glycans with the RP separation.
GlycoWorks RapiFluor-MS Reducing Protocol Provides a Rapid Streamlined Solution for Complex N-Glycan Analysis
The new reducing protocol is similar to the original GlycoWorks (non-reduced protein) protocol automated on the Andrew+ Pipetting Robot, except two reagents are introduced - Dithiothreitol (DTT) and ammonium acetate. DTT is used to reduce the disulfide bonds that can hinder the effectiveness of the PNGase F enzyme for some N-glycans. Ammonium acetate is used to minimize an interference peak when using mixed-mode reversed-phase columns such as the ACQUITY Premier Glycan BEH C18 AX column (Waters, p/n 186009758). Ammonium acetate is optional if other types of columns are used. Figure 1 shows an overview of the reduced protein protocol, which includes three steps: (1) Reduce and Deglycosylate, (2) Label and Quench, and (3) Clean up.
The N-linked glycans are released using the rapid reducing protocol. Briefly, a 10 µL volume of 12 μg glycoprotein solution was dispensed into a 96-well plate, followed by the addition of 10 μL of 3% (w/v) RapiGest SF and 2 μg/μL DTT denaturing buffer. After heat denaturation at 90°C for 3 min, the solution is cooled to ambient temperature and a 10 μL volume of Rapid PNGase F solution was added. Deglycosylation is continued at 50°C for 5 min. The resulting deglycosylated solution was labeled by adding 15 μL of RapiFluor-MS reagent. The labeling reaction was allowed to continue for 5 min at ambient temperature. Ammonium acetate solution (10 μL, 0.5 M) was added to quench the labeling reaction for 5 min at ambient temperature.
To prepare for the purification of N-linked glycans, a HILIC μelution plate was first conditioned with 200 μL of water and equilibrated with 200 μL of 85% acetonitrile. The resulting N-glycan mixture was diluted with 495 μL acetonitrile and loaded onto the plate. Samples were washed with four volumes of 300 μL of 1% formic acid, 90% acetonitrile to remove the impurities and excess labeling reagent. The glycans were eluted using three 30 μL volumes of elution buffer (total volume = 90 μL) and then diluted with 310 μL sample diluent to obtain purified released glycans.
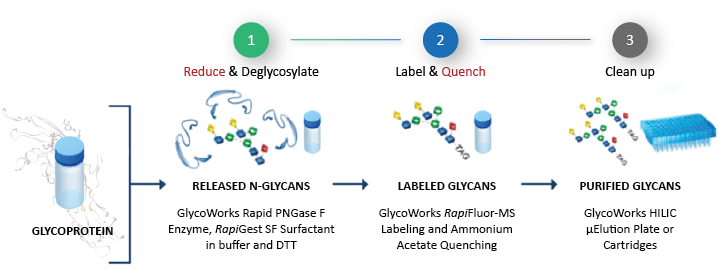
Figure 1: Workflow for the rapid preparation of N-glycan samples using the GlycoWorks RapiFluor-MS reducing protocol.
Automation of N-Glycan Analysis via GlycoWorks RapiFluor-MS Reducing Protocol
Automation of routine glycan analysis is highly suitable for the QC environment as well as general research for high consistency and reproducibility. The automated reduced protein GlycoWorks protocol can deliver a highly reproducible profile of complex glycans. Steps for the automated reduced protein protocol are shown in the flow diagram below.
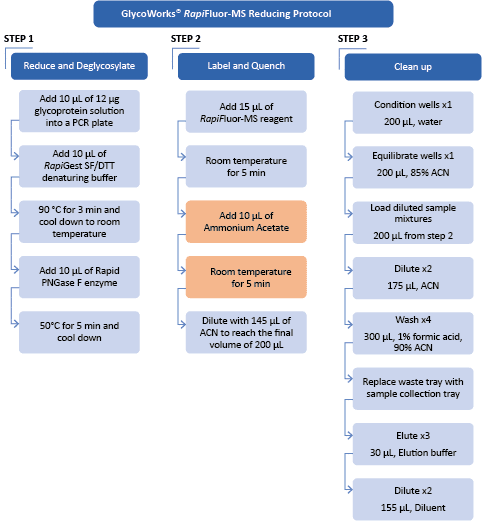
Figure 2: Flow diagram of the automated GlycoWorks RapiFluor-MS reducing protocol. Quenching with ammonium acetate is optional for HILIC separation.
For this procedure, the Andrew+ Pipetting Robot was placed inside a chemical hood (Model HBBV6; Dimensions 72W x 33.5D, 13.8 sq. ft.; Lab Crafters. Inc.). Two configurations of the Andrew+ working deck are shown in Figures 3 and 4. The protocol in Figure 3 uses three rows of Dominos/Devices+ and will execute the reducing protocol in its entirety. Users electing to work in a smaller chemical hood, such as the one above-mentioned, would need to use the two-step protocol shown in Figure 4 (steps A and B), with a configuration of two rows of Dominos/Devices+ each. "Step A" protocol will execute the reduction, deglycosylation, labeling, and quenching, while “Step B” will execute the clean-up.
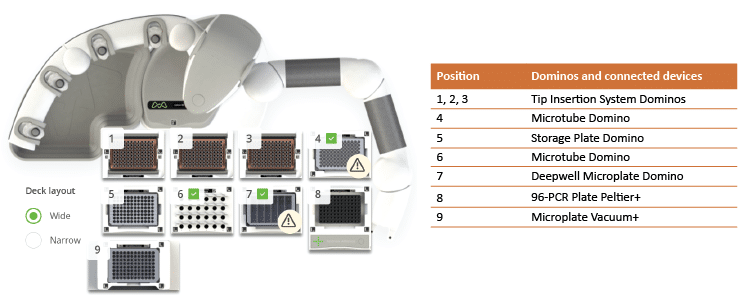
Figure 3: Andrew+ working deck configuration for the GlycoWorks RapiFluor-MS reducing 32-sample protocol. This configuration uses three rows of Dominos/Devices+. The execution time for 32 samples is 1 hour.
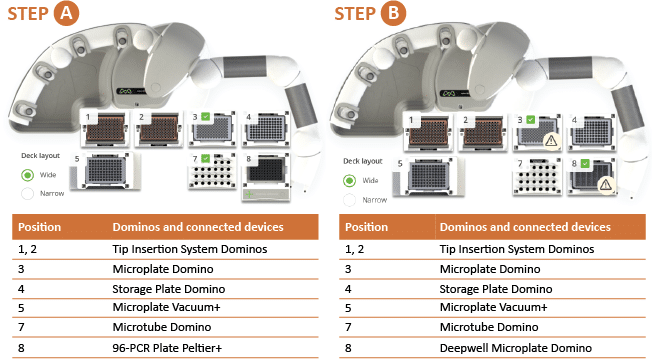
Figure 4: Andrew+ working deck configuration for the GlycoWorks RapiFluor-MS reducing 32-sample two-step protocol: Steps A and B. This configuration uses two rows of Dominos/Devices+ to accommodate a smaller chemical hood. The total execution time for 32 samples is 1 hour 10 min.
Rapid Automated Reducing Protocol Can Be Used for Profiling N-Glycans from Disulfide-Rich Glycoproteins
Human chorionic gonadotropin (hCG) was selected to test the reducing protocol due to its highly complex structure. The structure of hCG consists of a dimer representing two highly glycosylated subunits, and four N-linked glycans along with 11 disulfide bonds. The N-glycans attached are mostly of the complex type.
Reducing protocol enabled the release of N-glycans that are hard to access when using the non-reducing protocol. Figure 5 shows the difference in glycan profiles between the two protocols. The reduction with DTT before denaturing allowed more glycans to be accessible by the PNGase F enzyme for denaturing. The increased amount of glycans released was evident in peaks marked with (*) for the reducing protocol (Figure 5, black trace). The three peaks labeled with (*) were higher for the reducing protocol (Figure 5, black trace) than those labeled with (*) for the non-reducing protocol (Figure 5, pink trace).
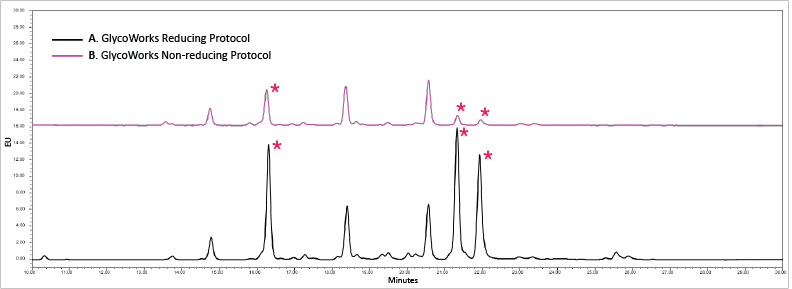
Figure 5: Glycans released from Human chorionic gonadotropin (hCG) using (A, black trace) the GlycoWorks reducing protocol and (B, pink trace) the GlycoWorks non-reducing protocol. The reducing protocol increased the amount of released glycans on the peaks marked with an asterisk (*).
Using the rapid automated reducing protocol, Andrew+ provided consistent glycan profiles released from hCG. Figure 6A compares the relative areas of the released glycans between the manual and automated executions of the protocol. Relative areas obtained for all nine glycans are comparable (%RSD of peak 5 and 6 ≤ 5%). For each of these sample preparations, six of the prepared samples were analyzed by UPLC.
Andrew+ can achieve improved reproducibility (%RSD of peak 5 and 6 <15%) of total areas of glycans. For each of these sample preparations, six of the prepared samples were analyzed by UPLC. Total areas obtained from the manual execution of the procedure (n = 6) and those from automated procedures (n = 6, 16, 32) are comparable as shown in Figure 6B.
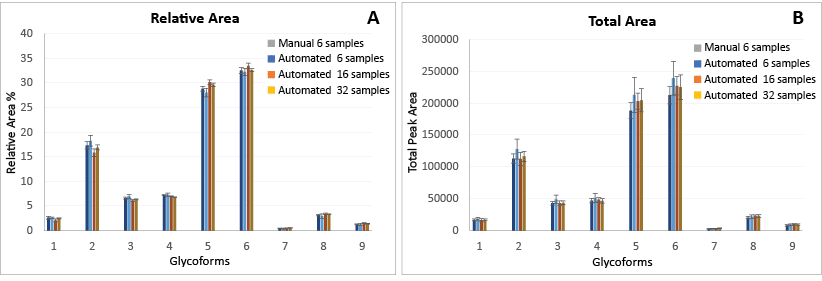
Figure 6: Comparison of glycan profiles obtained from the manual execution of the procedure (n = 6) and those from the automated procedure using the Andrew+ Pipetting Robot (n = 6, 16, and 32). Human Chorionic Gonadotropin (hCG) was used for these experiments.
Conclusions
Highly sensitive to manufacturing conditions, glycans are routinely monitored and characterized as a critical quality attribute (CQA) of protein biotherapeutics. Automation of routine glycan analysis is invaluable for general research as well as process and product development. The relative abundance, as well as recovery of released N-glycans in samples prepared on Andrew+, were comparable to those observed with manually executed experiments. In summary, we present an automated and complementary method that can provide reproducible glycan profiles from disulfide-rich glycoproteins with complex glycans such as hCG as demonstrated here.
ORDERING INFORMATION
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
➤ GlycoWorks Reducing Protocol - 6 Samples – Andrew+
- 1x Tip Insertion System Domino | p/n 186009612
- Microplate Domino | p/n 186009600
- Storage Plate Domino | p/n 186009596
- Microtube Domino | p/n 186009601
- Deepwell Microplate Domino | p/n 186009597
- 96-PCR Plate Peltier+ | p/n 176004584
- Microplate Vacuum+ | p/n 176004579
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 µL | p/n 186009606
- Sartorius, Optifit Tips, 5-350 µL (x46) | p/n 700013297
➤ GlycoWorks Reducing Protocol - 16 Samples – Andrew+
- 2x Tip Insertion System Domino | p/n 186009612
- Microplate Domino | p/n 186009600
- Storage Plate Domino | p/n 186009596
- Microtube Domino | p/n 186009601
- Deepwell Microplate Domino | p/n 186009597
- 96-PCR Plate Peltier+ | p/n 176004584
- Microplate Vacuum+ | p/n 176004579
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 µL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette: 8-ch 300 µL | p/n 186009607
- Sartorius, Optifit Tips, 5-350 µL (x144) | p/n 700013297
➤ GlycoWorks Reducing Protocol - 32 Samples – Andrew+
- 3x Tip Insertion System Domino | p/n 186009612
- Microplate Domino | p/n 186009600
- Storage Plate Domino | p/n 186009596
- Microtube Domino | p/n 186009601
- Deepwell Microplate Domino | p/n 186009597
- 96-PCR Plate Peltier+ | p/n 176004584
- Microplate Vacuum+ | p/n 176004579
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 µL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 µL | p/n 186009607
- Sartorius, Optifit Tips, 5-350 µL (x252) | p/n 700013297
Application Kits
Kit Components for the Andrew+ 24 Sample GlycoWorks Application | p/n 176003349
- GlycoWorks Rapid Deglycosylation Kit (3 x 8 Samples) by Waters | p/n 186008841
- GlycoWorks RapiFluor-MS Labeling Module (3 x 8 Samples) by Waters | p/n 186008091
- GlycoWorks HILIC μElution Plate by Waters | p/n 186002780
- GlycoWorks SPE Reagents – Automation by Waters | p/n 186008747
Kit Components for the Andrew+ 96 Sample GlycoWorks Application | p/n 176003350
- GlycoWorks Rapid Deglycosylation Kit (4 x 24 Samples) by Waters | p/n 186008840
- GlycoWorks RapiFluor-MS Labeling Module (4 x 24 Samples) by Waters | p/n 186007989
- GlycoWorks HILIC μElution Plate by Waters | p/n 186002780
- GlycoWorks SPE Reagents – Automation by Waters | p/n 186008747
Kit Components for the Andrew+ 96HT Sample GlycoWorks Application | p/n 176003351
- GlycoWorks Rapid Deglycosylation Kit (2 x 48 Samples) by Waters | p/n 186004579
- GlycoWorks RapiFluor-MS N-Glycan Kit – Automation (2 x 48 Samples) by Waters | p/n 186008822
- GlycoWorks HILIC μElution Plate by Waters | p/n 186002780
- GlycoWorks SPE Reagents – Automation by Waters | p/n 186008747
Reagents & Chemicals (Not provided in GlycoWorks kits)
- Intact mAb Mass Check Standard | p/n 186006552
- GlycoWorks RapiFluor-MS Glycan Performance Test Standard | p/n 186007983
Recommended Consumables
- Agilent 6-column reagent reservoir | p/n 201284-100
- Eppendorf twin.tec® 96-well skirted LoBind PCR Plate | p/n 0030129555
- Eppendorf 1.5 mL Safe-Lock tube | p/n 0030120086
- Waters QuanRecovery™ 700 μL 96-well plate | p/n 186009185
1) Varki, A.; Cummings, R. D.; Esko, J. D.; Stanley, P.; Hart, G. W.; Aebi, M.; Darvill, A. G.; Kinoshita,T.; Packer, N. H.; Prestegard, J. H.; Schnaar, R. L.; Seeberger, P. H. Essentials of Glycobiology, Third Edition. 2017.
2) Zhang, P.; Woen, S.; Wang, T.; Liau, B.; Zhao, S.; Chen, C.; Yang, Y.; Song, Z.; Wormald, M. R.; Yu, C.; Rudd, P. M. Challenges of glycosylation analysis and control: an integrated approach to producing optimal and consistent therapeutic drugs. Drug Discovery Today. 2016, 21 (5), 740-765.
3) Hires, M.; Jane, E.; Mego, M.; Chovanec, M.; Kasak, P.; & Tkac, J. Glycan Analysis as Biomarkers for Testicular Cancer. Diagnostics (Basel, Switzerland), 2019, 9(4), 156.
4) Biskup, K., Blanchard, V., Castillo-Binder, P., Alexander, H., Engeland, K., & Schug, S. (2020). N- and O-glycosylation patterns and functional testing of CGB7 versus CGB3/5/8 variants of the human chorionic gonadotropin (hCG) beta subunit. Glycoconjugate journal, 2020, 37(5), 599–610.
5) Lauber, M. A.; Yu, Y.-Q.; Brousmiche, D. W.; Hua, Z.; Koza, S. M.; Magnelli, P.; Guthrie, E.; Taron, C. H.; Fountain, K. J. Rapid Preparation of Released N-Glycans for HILIC Analysis Using a Labeling Reagent That Facilitates Sensitive Fluorescence and ESI-MS Detection. Anal. Chem. 2015, 87 (10), 5401–5409.
6) Koza, S. M.; McCall, S. A.; Lauber, M. A.; Chambers, E. E. Quality Control and Automation Friendly GlycoWorks RapiFluor-MS N-Glycan Sample Preparation (720005506EN). Waters Corporation May 2020
7) Reed, C. E., Koza, S., Calciano, S. Automated Medium and High-Throughput GlycoWork RapiFluor-MS Preparations on the Andrew+ Pipetting Robot. (720007008EN). Waters Corporation September 2020
8) GlycoWorks RapiFluor-MS Quick Start Protocol for Disulfide Rich Glycoproteins - 24 Samples. (720006992EN). Waters Corporation April 2021
9) GlycoWorks RapiFluor-MS Quick Start Protocol for Disulfide Rich Glycoproteins - 96 Samples. (720006991EN). Waters Corporation December 2020
10) Lauber, M. A.; Brousmiche, D. W.; Hua, Z.; Koza, S. M.; Guthrie, E.; Magnelli, P.; Taron, C. H.; Fountain, K. J. Rapid Preparation of Released N-Glycans for HILIC Analysis Using a Novel Fluorescence and MS-Active Labeling Reagent. (720005275EN). Waters Corporation September 2020
Protocols
Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 