or start from open source methods. Learn more about OneLab softwareUse OneLab
GlycoWorks® N-Glycan Automated Sample Prep

This advanced method offers an end-to-end solution developed around an advanced application, a specialized kit, or an analytical system. It delivers complete functional, ready-to-use protocols that are qualitatively and quantitatively assessed for consistency, executability, and repeatability, usually without needing to make any changes. It operates per batch size and is scalable to accommodate changing requirements.
Overview
Protein Glycosylation is Fundamental for Protein Function and Cell Physiology
Glycosylation is an essential form of post-translational modification (PTM) of human and eukaryotic proteins, which consists of the covalent attachment of a glycan moiety (saccharides or sugar chains) to the protein surface. Glycans are characterized by their composition and structural diversity and are found in cell-surface and secreted proteins (Figure 1). They contribute to the conformation and biological function of proteins and their binding characteristics, stability, and solubility. Glycosylated proteins, also known as glycoproteins, are involved in various biological processes.
There is a growing interest in the study of protein glycosylation dynamics, as aberrant glycosylation patterns have been associated with various human pathologies (1). Therefore, the identification and quantification of protein glycans have become increasingly important in basic research to understand their role in protein regulation and function, both under normal and pathological conditions, and in biotechnology to ensure efficient production of recombinant glycoproteins for therapeutic applications.
Glycosylation is Routinely Monitored for the Development of Therapeutically Safe and Effective Biologics
Many recombinant biopharmaceuticals, such as monoclonal antibodies (mAbs), are glycoproteins. Bioprocessing requires precise control and monitoring of multiple parameters, including cell line stability, product yield, protein folding, and PTMs. Among these parameters, the host cell’s biosynthesis of glycans is routinely monitored as a critical quality attribute (CQA). Glycan composition and structure are crucial for the biological or clinical activity of the drug. Changes in manufacturing conditions can alter the glycosylation patterns of recombinant proteins and, consequently, the end drug product's biological activity, safety, stability, efficacy, and immunogenicity (2). Therefore, the manufacturing of therapeutic glycoproteins requires careful monitoring and characterization of their glycosylation profile (e.g. content, structure, abundance, etc...) to achieve consistent glycan composition and meet desired quality, clinical safety, and effectiveness targets.
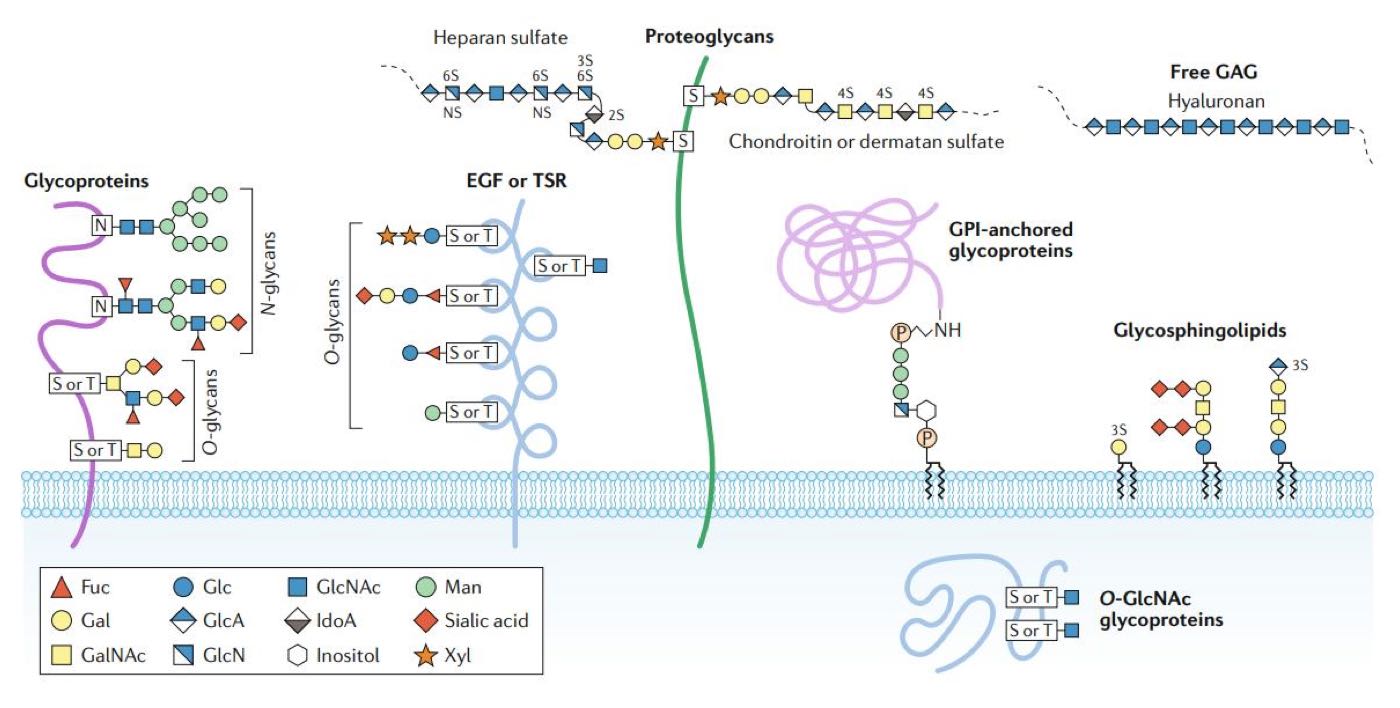
Figure 1: Major forms of glycosylation in human cells (1). Glycans are covalently attached to proteins (and lipids) to form glycoconjugates. Glycans are classified according to the linkage to the protein, glycan (or lipid) moieties. Glycoproteins consist of glycans, and branched glycan chains linked to nitrogen or oxygen atoms of amino acid residues. Glycans bound to the oxygen atom of the hydroxyl groups of Serine (Ser, S) or Threonine (Thr, T) are termed O-glycans. Glycans linked to the nitrogen atom of the amino group of Asparagine (Asn, N) are known as N-glycans. Fuc, Fucose; Gal, Galactose; GalNAc, N-acetylgalactosamine; Glc, Glucose; GlcA, Glucuronic acid; GlcN, Glucosamine; GlcNAc, N-acetylglucosamine; IdoA, iduronic acid; Man, Mannose; Xyl, Xylose; EGF, Epidermal growth factor; TSR, Thrombospondin type I repeats; GPI, Glycosylphosphatidylinositol; GAG, Glycosaminoglycan.
Analysis of Protein N-Glycans and Challenges
N-linked glycosylation is a very common protein modification in eukaryotic cells. It refers to the attachment of glycans to the amide group in the side chain of asparagine (Asn, N) residues of newly synthesized proteins. The N-Glycosylation of biopharmaceuticals must be maintained during production, as the N-Glycan profile of recombinant proteins measures efficacy, quality, bioactivity, and optimal manufacturing conditions.
Several analytical approaches exist for analyzing and characterizing protein N-glycans during process development. One commonly used approach involves the enzymatic release of N-Glycan chains from the protein of interest and their derivatization with a fluorescent label. Subsequently, labeled N-Glycans are analyzed using chromatographic methods and detected using fluorescence measurement and potentially mass spectrometry (MS). Hydrophilic interaction liquid chromatography (HILIC), ideally used for the separation of highly polar compounds, has proven to be a robust and reliable technique that effectively separates and quantitates fluorescently labelled N-glycans (3, 4).
Conventional workflows for N-glycan HILIC analysis can be laborious and time-consuming, including a long incubation time and cumbersome labeling reactions, sometimes resulting in labeled glycans that are challenging to detect using fluorescence or MS (4). Therefore, there is a need for a more rapid sample preparation method, allowing for improved sample throughput while ensuring selective and highly sensitive N-glycan profiling of recombinant glycoproteins.
Accelerating the sample preparation of N-glycans and facilitating their detection is essential for routine monitoring of drug product consistency throughout biopharmaceutical process development and manufacturing. Precise N-glycan composition and structure data enable operators to intervene at critical control points in bioprocessing to reduce risks and ultimately ensure the delivery of safe and effective biotherapeutics.
Rapid Preparation of N-Glycans using Waters GlycoWorks RapiFluor-MS N-Glycan Kit
The GlycoWorks RFMS N-Glycan kit is the best combination of speed and simplicity, delivering enhanced fluorescence response (FLR) and mass spectrometric (MS) sensitivity for the detection of released N-linked glycans. The core technology of this sample preparation approach is the use of an innovative, proprietary labeling reagent named RapiFluor-MS (or RFMS), which rapidly reacts with N-glycans following their enzymatic release from glycoproteins (4, 5). RFMS is a highly reactive, primary/secondary amine labeling reagent. It comprises an N-hydroxysuccinimide (NHS)-carbamate reactive group, a quinoline fluorophore, and a basic tertiary amine (Figure 2). As a result, RFMS-labeled glycans can be detected by fluorescence and positive ion mode electrospray ionization (ESI)-MS with high sensitivity.
Reagents and reaction conditions used in this kit have been optimized to enable a fast and complete de-N-glycosylation of glycoproteins and, subsequently, rapid, and efficient labeling of released N-glycans with RFMS. The acceleration of the entire N-glycan sample preparation workflow directly results from integrating these two procedures with a HILIC solid-phase extraction (SPE) sample clean-up step. HILIC SPE purification provides quantitative recovery of RFMS-labeled N-glycans. It allows for immediate analysis of prepared samples using hydrophilic interaction liquid chromatography with fluorescence detection (HILIC-FLR) and potentially mass spectrometry for further characterization or mass confirmation.
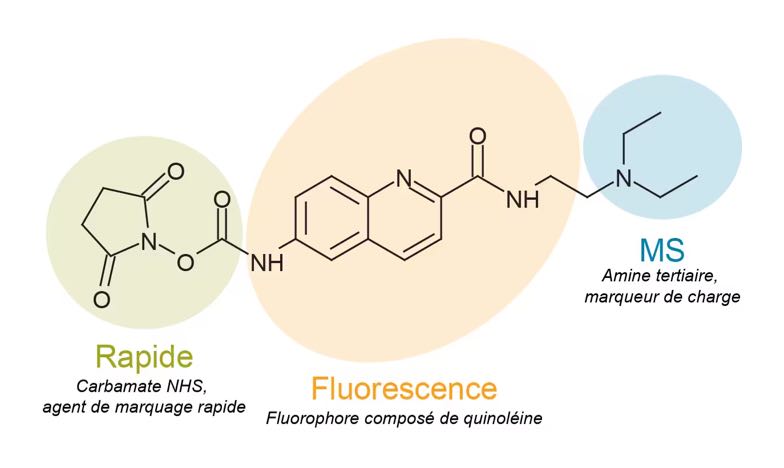
Figure 2: Structure of the RapiFluor-MS (RFMS) labeling reagent. The features of the chemical structure that enable rapid tagging of N-glycans, efficient fluorescence response, and enhanced MS sensitivity are highlighted (5).
Experimental Procedure
The N-glycan sample preparation workflow comprises three steps: The release, labeling, and purification of N-glycans. To prepare N-glycans for labeling, glycoprotein samples (15 μg) are first denatured using RapiGest SF, an enzyme-friendly anionic surfactant, reconstituted in GlycoWorks Rapid Buffer. Mixtures are heated to approximately 90°C for 3 min, allowed to cool to room temperature, and subsequently mixed with the peptide-N-glycosidase F (GlycoWorks Rapid PNGase F). Heating samples to a temperature of at least 90°C is crucial to ensuring that glycoproteins are sufficiently denatured and that N-glycans are readily accessible to the PNGase F enzyme (4). The de-N-glycosylation reaction is completed by incubating the samples at 50°C for 5 min. In this approach, glycoproteins are efficiently deglycosylated in approximately 10 min to produce N-glycosylamines from a diverse set of glycoproteins. Released N-glycosylamines exhibit a relatively stable structure in the applied deglycosylation conditions (4).
The deglycosylation mixtures are allowed to cool to room temperature following their incubation at 50°C and then rapidly reacted with RFMS labeling reagent, dissolved in anhydrous dimethylformamide (DMF) or dimethylsulfoxide (DMSO), without a protein depletion step. This labeling procedure occurs at room temperature in 5 minutes, a major improvement over the multiple hours typically required for this process using traditional N-glycan labels (4, 6). To facilitate MS ionization, labelled N-glycosylamines bear both a fluorescent tag comprised of an efficient fluorophore and a basic tertiary amine motif. Labeling reactions are finally diluted with acetonitrile (ACN) in preparation for HILIC SPE clean-up.
The final step of the workflow is the purification and enrichment of the RFMS-labeled N-glycans through a robust HILIC SPE method. Wells containing 5 mg sorbent are conditioned with water and equilibrated with 85% (v/v) ACN before loading samples. Adsorbed samples are subsequently washed twice with a solution containing 1% formic acid in 90% ACN to remove potential interferences, such as labeling reaction byproducts from RFMS-labeled N-glycans as well as an excess labeling reagent. Finally, after replacing the waste collection tray with the sample collection plate, enriched, RFMS-labeled N-glycosylamines are eluted from the SPE sorbent with three 30 μL volumes of GlycoWorks SPE Elution Buffer composed of 200 mM ammonium acetate in 5% ACN. In preparation for analysis, the SPE eluate is diluted with 310 μL of the GlycoWorks Sample Diluent (DMF or DMSO/ACN mix, 32/68%). DMF of DMSO/ACN diluted samples can be immediately analyzed with the ACQUITY UPLC Glycan BEH Amide 130 Å, 1.7 μm column (Waters, p/n 186004742) on a well-configured ACQUITY UPLC system.
Moving Towards a Safer and Environmentally Friendly Sample Preparation
Anhydrous dimethylformamide (DMF) solvent has been restricted by the 🇪🇺 European Chemicals Agency (ECHA) because of its negative effects on workers. To address this and offer a more environmentally friendly alternative, dimethyl sulfoxide (DMSO) solvent was studied as a substitute in the RapiFluorMS labeling reaction and final dilution steps prior to GlycoWorks N-Glycan analysis.
Waters' studies have shown that DMSO can be used as an alternative to DMF in the RapiFluor-MS N-Glycan GlycoWorks procedure (11). Comparable solubilization of RapiFluor-MS with anhydrous DMSO and subsequent RFMS labeling was demonstrated. DMSO, was also found to be effective as a component in the sample diluent used for RFMS-labeled and HILIC SPE purified N-glycans. Some minor considerations need to be taken into account regarding injection volume when however, maximum injection volumes for amide HILIC analysis are slightly reduced for DMSO versus DMF diluted samples. As a result, injection volumes when using DMSO as a diluent component may need to be reduced appropriately.
Automation of the GlycoWorks RFMS Protocol on the Andrew+ Pipetting Robot
During the process of development and manufacturing of biopharmaceutical products, it may be necessary to screen large numbers of samples, which can be time-consuming and monotonous. Additionally, robust and reproducible methods for glycan preparation and analysis are a prerequisite for highly regulated glycoprotein-based biotherapeutics to ensure accuracy and consistency.
Standardization of glycan sample preparation is essential to minimize variability by reducing manual pipetting errors, and to ensure that glycan data is accurate and reproducible. One approach to achieving this is through the integration of automation to streamline routine sample preparation, either using a fully automated liquid handling system such as the Andrew+ pipetting robot, improving both reproducibility and sample throughput or the Pipette+ guided pipetting system, providing real-time step-by-step guidance throughout the experiment to minimize pipetting errors.
Watch this short video to see how Andrew+ simplifies
the GlycoWorks workflow through automation.
Note: Extraction+ vacuum pump replaces Vacuum+ (shown in the video) to significantly reduce user actions and improve productivity.
The protocol used for the implementation of the GlycoWorks RFMS kit on Andrew+ is the quality control/automation-friendly sample preparation method (7). The quality con¬trol (QC) protocol is an adaptation of the standard variable volume (VV) method, in which the starting sample concentration and reagent concentrations used during protein denaturation, deglycosyl-ation, and N-glycan labeling, have been carefully modified to allow pipetting volumes of 10 μL or more thus improving pipetting accuracy and generating results comparable to those produced by the standard VV method (7).
The QC/automation-friendly GlycoWorks protocol was demonstrated to be fit for purpose on Andrew+. Experimental conditions and parameters were fine-tuned to fit the hardware configuration of the Andrew+ robot, i.e. labware Dominos and required additional connected devices (Figure 3), and produce reliable results comparable to target scores and reproducibility among users. The Andrew+ robot can also greatly simplify the process of normalizing the concentrations of protein samples to meet the requirements of the GlycoWorks RFMS protocol.
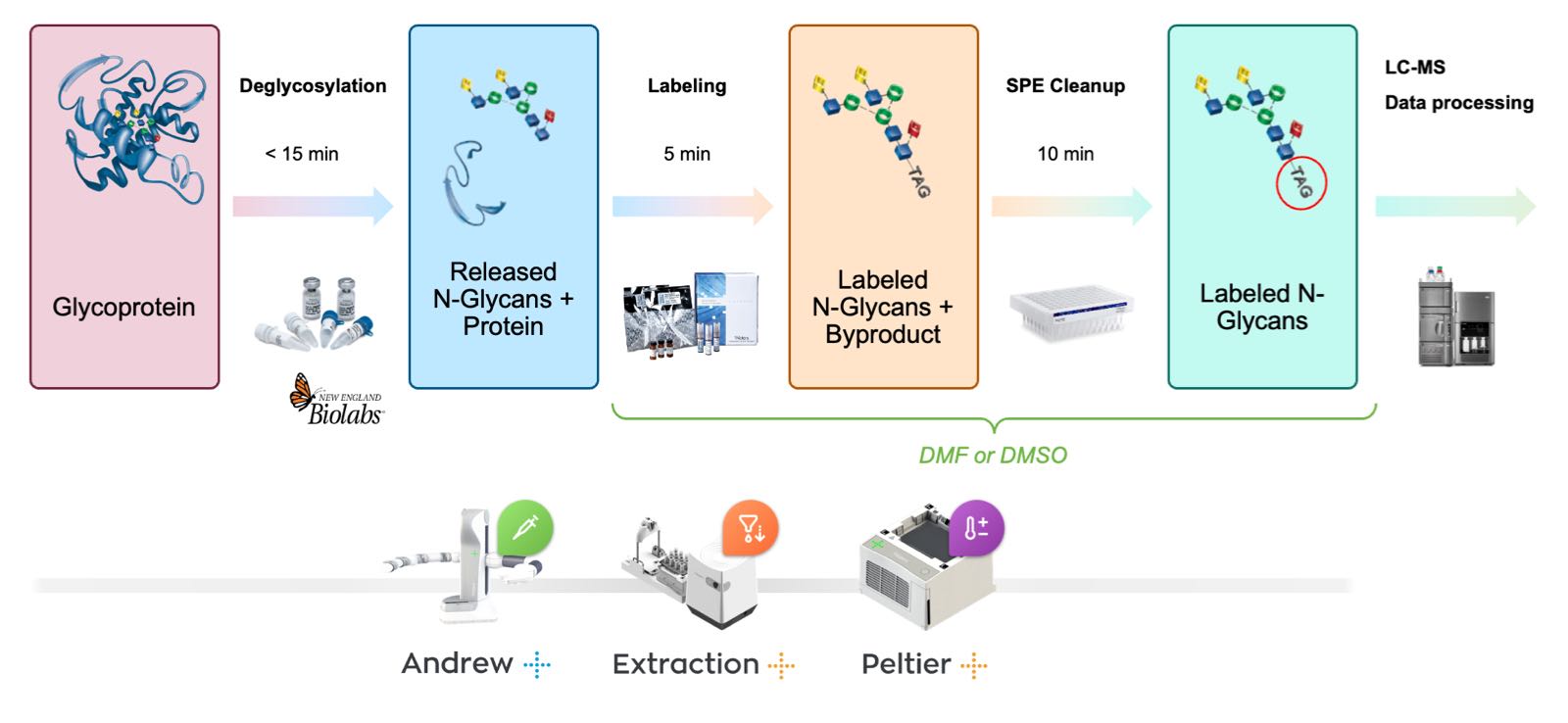
Figure 3: Flow diagram for the automated rapid preparation of N-glycans samples using the GlycoWorks RapiFluor-MS N-glycan kit and the Andrew+ Pipetting Robot.
Note: This procedure offers the flexibility to automate the protocol with either a Vacuum+ or an Extraction+ vacuum pump, enabling a higher degree of automation with the latter. The two different setups have been thoroughly examined, and each has proven to be highly effective in preparing released and labeled N-glycan samples. These setups offer a range of automation options, providing researchers with flexibility in their sample preparation processes.

Figure 4: Andrew+ deck layout and pipette/tool selection for the automated protocol "GlycoWorks Sample Preparation, 48 Samples - Andrew+ with Peltier+ and Extraction+".
Case study: Preparation of Released and Labeled N-Glycans from Protein A-Purified mAbs for High-Throughput Analysis on Waters BioAccord System
Protein A purification is often used in drug development to purify biotherapeutic mAbs. When glycine and tris are used to elute and neutralize Protein-A purified mAbs, buffer exchange is required prior to GlycoWorks sample preparation due to the incompatibility of RFMS with high concentrations of nucleophiles (see Consideration #2 below). To accommodate scenarios in which mAbs are prepared in nucleophilic buffers, a high throughput diafiltration step was incorporated into the automated GlycoWorks RFMS protocol.
The GlycoWorks Diafiltration 48-Sample protocol was optimized and demonstrated fit for purpose by Waters on the Andrew+ Pipetting Robot using the connected Extraction+ Vacuum system. Due to space constraints, a two-step protocol is employed wherein Step 1 executes diafiltration, deglycosylation, and labeling (Figure 5) and Step 2 executes the SPE purification (Figure 6). The protocol can prepare released and labelled N-glycans from 48 protein A-purified samples in three hours. When coupled with a five-minute method on the BioAccord LC-MS system, the protocol can deliver reproducible glycan profiles of 48 mAb samples in four hours and quantify abundant and trace-level N-glycans. Importantly, this method may be adapted for samples prepared at any concentration in any buffer, regardless of RFMS compatibility, and has potential use for other glycoproteins.

Figure 5: Flow diagram outlining the automated diafiltration GlycoWorks RapiFluor-MS protocol for rapid preparation of labeled N-glycans from mAb samples at low concentration or in nucleophilic buffer.
Step 1a + 1b - Diafiltration, Deglycosylation and Labeling

Figure 6: Andrew+ deck layout and pipette/tool selection for the automated protocol "GlycoWorks Sample Prep – Steps 1a and 1b, with Diafiltration – 48 samples".
Step 2 - Cleanup

Figure 7: Andrew+ deck layout and pipette/tool selection for the automated protocol "GlycoWorks Sample Prep – Step 2, Cleanup – 48 samples".
Considerations
1. Recommended Starting Glycoprotein Quantity
- Each reaction in The GlycoWorks RFMS N-glycan protocol is designed to produce optimal results from 15 μg of glycoprotein. The QC method uses 10 μL of glycoprotein sample at a concentration of 1.5 mg/mL (Figure 4). Samples with a concentration slightly higher or lower than 1.5 mg/mL can produce an appropriate output. Significant changes to the optimal glycoprotein quantity, i.e. < 5 μg and > 40 μg, can affect the PNGase F enzyme to substrate ratio as well as the molar excess of RFMS labeling reagent, which will potentially result in undesirable labeling artefacts or low yield (7). If samples with concentrations significantly lower than 1.5 mg/mL are used, the GlycoWorks Sample Prep with Diafiltration 48-sample protocol can be adapted to concentrate the sample during the diafiltration step.
2. Buffer/Formulation Considerations
- The rapid deglycosylation reaction, facilitated by the RapiGest SF surfactant, can be compromised by the presence of sodium dodecyl sulfate (SDS) in the sample. Moreover, the presence of nucleophiles at high concentrations can compromise the N-glycan labeling reaction. Therefore, the concentration of amine and/or thiol-containing compounds should be diluted to a final concentration of < 0.1 mM. On the other hand, a buffer exchange step could be necessary for some samples before enzymatic deglycosylation. In this case, it is recommended to exchange the protein sample for a neutral sodium phosphate, citrate, or ideally a 50 mM HEPES buffer (free acid, titrated to pH 7.9 with sodium hydroxide).
- To perform a buffer exchange or to prepare samples out of complex matrices (lysates/biofluids), consider the use of molecular weight cut-off (MWCO) filtration, dialysis, or protein precipitation (i.e., ethanol precipitation). With these techniques, it is recommended to exchange a protein sample for a neutral sodium phosphate, citrate, or HEPES buffer. A matching formulation to the GlycoWorks Rapid Buffer would be 50 mM HEPES-free acid titrated to pH 7.9 with sodium hydroxide.
- Alternatively, the GlycoWorks Sample Prep with Diafiltration 48-sample protocol can be used to prepare released and labelled glycans from samples in nucleophilic buffers. This protocol includes a high-throughput MWCO diafiltration step prior to N-glycan release and labeling.
3. Ensuring Complete Denaturation & Deglycosylation
- It is imperative that the glycoprotein be subjected to heat denaturation prior to the addition of PNGase F. In the heat denaturation step, ensure that the glycoprotein is subjected to a temperature of at least 90°C. Some challenging samples may require such a high temperature and possibly even near-boiling conditions (100°C) in order for complete deglycosylation to be achieved.
4. Regarding the RapiFluor-MS Reagent
- RapiFluor-MS is purified as a 1:1 complex with NHS. The formula weight for the reagent as provided is 542.41 g/mol.
- RapiFluor-MS is a highly reactive, primary/secondary amine labeling reagent. It hydrolyzes in water with a half-life of the order of 10-100 seconds (see Figure 4). It is, therefore, important that the reagent be dissolved in the provided anhydrous DMF or DMSO, non-nucleophilic, polar aprotic solvents. Reagent solution can be used across the span of a day if care has been taken to limit exposing the solution to atmospheric moisture.
- Glycans are released from glycoproteins as glycosylamines, an important fact for an analyst to consider when using this labeling chemistry.
Protocol specifications
GlycoWorks Sample Prep – 48 samples:
- Estimated time of execution: 57m 9s
- Tip consumption:
- 203× 5-350 μL tips
- 32× 50-1200 μL tips
GlycoWorks Sample Prep – Steps 1a and 1b, with Diafiltration – 48 samples:
- Estimated time of execution: 1h 28m 11s
- Tip consumption:
- 84× 5-350 μL tips
GlycoWorks Sample Prep – Step 2, Cleanup – 48 samples:
- Estimated time of execution: 26m 55s
- Tip consumption:
- 112× 5-350 μL tips
Ordering information
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
- Andrew+ Pipetting Robot
- OneLab software
- 4× Tip Insertion System Domino | p/n 186009612
- Microtube Domino | p/n 186009601
- Microplate Domino | p/n 186009600
- Extraction+ Base Kit with Plate Gripper | p/n 176005201
- Extraction+ Plate Kit including 1× Deepwell Microplate Domino and 1× Storage Plate Domino | p/n 176005203
- 96-PCR Plate Peltier+ | p/n 176004584
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 µL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 µL | p/n 186009607
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 1200 µL | p/n 186009615
Additional suggested items
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 5000 µL | p/n 186009608
Application kits options
- Andrew+ 24-Sample GlycoWorks Eco Application Kit | p/n 176005291
- Andrew+ 96-Sample GlycoWorks Eco Application Kit | p/n 176005295
- Andrew+ 96HT Sample GlycoWorks Eco Application Kit | p/n 176005296
Recommended consumables
- Sartorius, Optifit Tips, 5-350 μL (x203) | p/n 700013297
- Sartorius, Optifit Tips, 50-1200 μL (x32) | p/n 700013300
- Waters QuanRecovery™ 700 μL 96-well plate | p/n 186009185
- AcroPrep™ Advance 350 μL 96-well filter plate, 10K Omega membrane | p/n 8034
- Eppendorf twin.tec® 96-well skirted LoBind® PCR plate | p/n 0030129555
- Axygen® 12-well reservoir, 12-channel trough | p/n RES-MW12-HP
References
- Reily C., Stewart T. J., Renfrow M. B., Novak J. (2019). Glycosylation in Health and Disease. Nat. Rev. Nephrol. 15, 346–366. 10.1038/s41581-019-0129-4 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6590709/)
- Fournier J. A Review of Glycan Analysis Requirements. BioPharm International. 2015; 28(10): 32–37 (https://www.biopharminternational.com/view/review-glycan-analysis-requirements)
- Tharmalingam T, Adamczyk B, Doherty MA, Royle L, Rudd PM. Strategies for the profiling, characterisation and detailed structural analysis of N-linked oligosaccharides. Glycoconj J. 2013;30(2):137–146. Epub 2012/08/28 (https://pubmed.ncbi.nlm.nih.gov/22922975/)
- Lauber MA, Yu Y-Q, Brousmiche DW, Hua Z, Koza SM, Magnelli P, Guthrie E, Taron CH, Fountain KJ. Rapid Preparation of Released N-Glycans for HILIC Analysis Using a Labeling Reagent that Facilitates Sensitive Fluorescence and ESI-MS Detection. Analytical Chemistry. 2015; 87(10):5401–5409 (https://pubmed.ncbi.nlm.nih.gov/25927596/)
- Waters Application Note 720005275EN - Rapid Preparation of Released N-Glycans for HILIC Analysis Using a Novel Fluorescence and MS-Active Labeling Reagent (https://www.waters.com/webassets/cms/library/docs/720005275en.pdf)
- Ruhaak LR, Zauner G, Huhn C, Bruggink C, Deelder AM, Wuhrer M. Glycan labeling strategies and their use in identification and quantification. Analytical and Bioanalytical Chemistry. 2010; 397(8):3457–3481 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2911528/)
- Waters Application Note 720005506EN - Quality Control and Automation Friendly GlycoWorks RapiFluor-MS N-Glycan Sample Preparation (https://www.waters.com/webassets/cms/library/docs/720005506en.pdf)
- Waters Application Note 720006971EN - GlycoWorks RapiFluor-MS Automation Using the Andrew+ Pipetting Robot and Vacuum+ Device (https://www.waters.com/webassets/cms/library/docs/720006971en.pdf)
- Waters Application Note 720007008EN - Automated Medium and High-Throughput GlycoWorks RapiFluor-MS Preparations on the Andrew+ Pipetting Robot Equipped with the Vacuum+ Device (https://www.waters.com/content/dam/waters/en/app-notes/2020/720007008/720007008-en.pdf)
- Waters Application Note 720007854EN - Automated High-Throughput N-Glycan Labeling and LC-MS Analysis for Protein A Purified Monoclonal Antibodies Using Andrew+ Pipetting Robot and Extraction+ Vacuum Pump (https://www.waters.com/content/dam/waters/en/app-notes/2023/720007854/720007854-en.pdf)
- Waters Application Note 720008157EN - Replacing DMF with DMSO in the Eco GlycoWorks™ RapiFluor-MS™ Labeled N-Glycan Sample Preparation (https://www.waters.com/content/dam/waters/en/app-notes/2023/720008157/720008157-en.pdf)
- GlycoWorks Kits product page (https://www.waters.com/nextgen/us/en/pro
Protocols





Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 