or start from open source methods. Learn more about OneLab softwareUse OneLab
Fungitell® Glucan Detection Assay
This advanced method offers an end-to-end solution developed around an advanced application, a specialized kit, or an analytical system. It delivers complete functional, ready-to-use protocols that are qualitatively and quantitatively assessed for consistency, executability, and repeatability, usually without needing to make any changes. It operates per batch size and is scalable to accommodate changing requirements.
Overview
Use of the Andrew+ Robot and OneLab to Automate Liquid Handling for the Fungitell® Glucan Detection Assay
Fungitell® is an FDA-cleared and CE-marked in-vitro diagnostic for the detection of (1→3)-β-D-glucan (BDG) in the serum of patients with symptoms of, or medical conditions predisposing the patient to, invasive fungal disease (1). The Fungitell® assay is a 96-well microplate-based assay in which the main components of the reagent are derived from the hemolymph of the horseshoe crab Limulus Polyphemus (2). The method takes advantage of the exquisite specificity of the pathogen-associated molecular pattern recognition proteins (PAMPS) within the horseshoe crab hemolymph to detect the fungal biomarker by recognition of the (1→3)-β-D bonds of the glucose biopolymer (Figure 1) (3,4). Glucans come from, among other sources, the cell surface of various medically important fungi (5). Their presence in serum is an indicator of potential fungal infection (6,7).
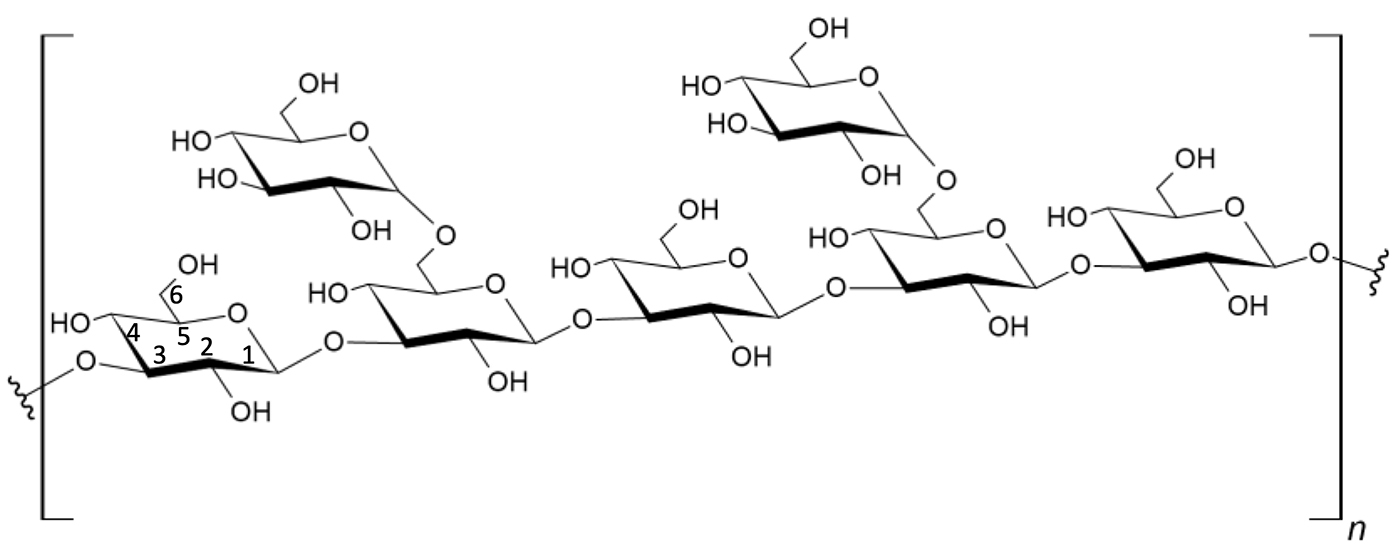
Figure 1: Common (1→3)-β-D-glucan structure. The example shown is a (1→3)-β-D-glucan polymer chain with (1→6)-β-D-glucan branching. Glucan is a major constituent of the cell wall of most Fungi for example, similar molecules can be derived from Saccharomyces cerevisiae cell walls.
The Fungitell® assay is extremely sensitive and can detect minute quantities of (1→3)-β-D-Glucan down to ~30 pg/mL (picograms (10-12 g/mL) (1). As indicated above, the assay uses the natural biosensing capability of protein zymogens derived from the horseshoe crab formulated with a chromogenic peptide. The time-based (kinetic) development of colour is observed using a spectrophotometric plate reader, typically with detection at 405 nm and at 495 nm. The development of colour over time is proportional to the amount of glucan in the sample compared to the standard curve.
Conducting the assay is an involved process necessitating numerous pipetting and arraying steps. Standard curve preparation, control, sample preparation (and pretreatment), and reagent dispensing are time-consuming and require attention to detail. Automation of these liquid handling steps significantly reduces tedium for these assays. Utilization of the Andrew+ Robotic System reduces the required pipetting steps by over 86% per plate, on average. The OneLab protocol provided below represents a basic procedure for the execution of the Fungitell® assay using triplicate standards, negatives, and controls with quadruplicate samples. Example results using Andrew+ are presented for standard curves and controls in Figures 2 and 3 below, respectively. As can be readily observed by the associated analyses, the reproducibility of the standard curves and the control samples is excellent.
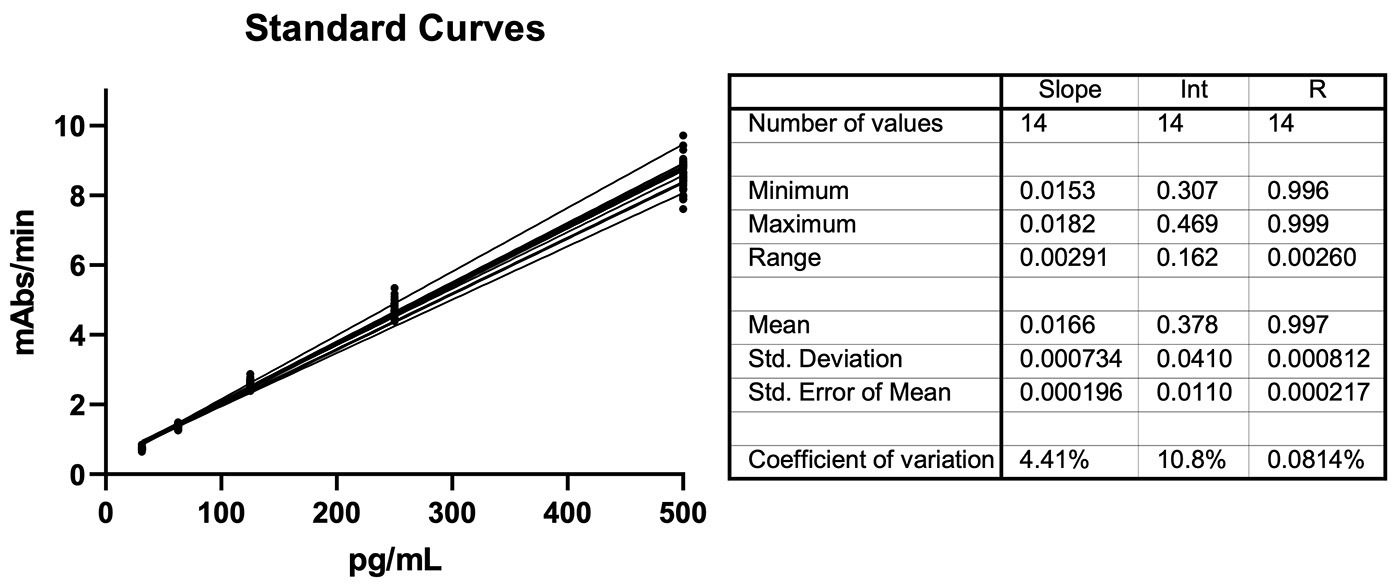
Figure 2: Example Fungitell® standard curves created by executing the protocol on the Andrew+ Pipetting Robot. There are fourteen separate standard curves included in this graph. Each curve has triplicates for each standard resulting in forty-two data points at each x-axis value. The 14 plates were collected by two people over 4 or more days using the Andrew+ robot. The standard curve comprises five points, 31.25, 62.5, 125, 250, and 500 pg/mL created by 2-fold serial dilution using Andrew+.
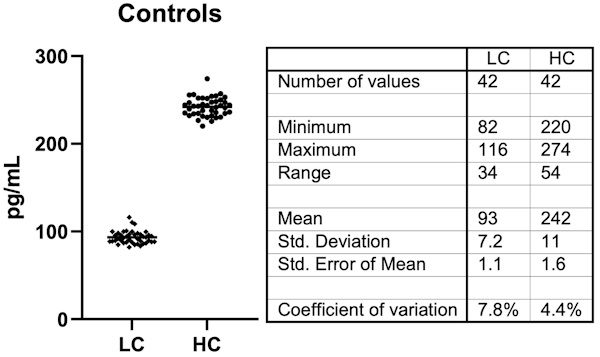
Figure 3: Example Fungitell® glucan serum control results using Andrew+. Controls were tested on each of the plates in Figure 2 in triplicate. The aggregate set of forty-two data points for each control was analyzed. LC is low control and HC is high control. Controls are serum-based samples spiked with glucan. A control or sample in this assay is created from three separate pipetting steps of 5, 20 and 100 µL in a single well.
Assay notes
Fungitell® assay execution note:
The Fungitell® assay can detect minute quantities of glucan in the picogram per millilitre range (10-12 g/mL). As indicated in the instructions for use (IFU), all materials used for assay execution must be tested for potential interferences. Excellent lab technique and a clean environment are required to execute these assays. It is suggested that all manipulations be carried out in a laminar flow hood or static enclosure to address these requirements.

Figure 4: Andrew+ OneLab deck setup for the Fungitell® assay. Samples and reagents locations on the Andrew+ deck: [1-2] Tips, [3] 96 well microplate, [4] Samples, Standards, APS/LRW, 1.5 mL conical tubes, [5] LRW/APS Bulk, 15 mL centrifuge or 16x90mm glass tubes, [6] Fungitell 10 mL reservoirs. Note: LRW is LAL reagent water, and APS is an alkaline pretreatment solution.
Ordering information
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
- Andrew+ Pipetting Robot
- OneLab software
- Microtube Domino | p/n 186009601
- Microplate Domino | p/n 186009600
- 16×90mm Glass Tube Domino | p/n 186010159
- 8-Channel Pipette Reservoir | p/n 186009613
- 2x Tip Insertion System Domino | p/n 186009612
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 10 μL | p/n 186009769
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
Additional suggested items
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 1200 μL | p/n 186009615
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 5 mL | p/n 186009608
- Tip Insertion System Domino | p/n 186009612
- 15mL Conical Centrifuge Tube Domino| p/n 186010087
- Microtube Domino | p/n 186009601
Recommended consumables
- ACC, Pyroclear® Pyroplate® 96-well microplate | p/n CA961-50
- INTEGRA 10 mL multichannel reservoir | p/n 4332
- Eppendorf 1.5 mL Safe-Lock tube | p/n 0030120086
- ACC, Pyrotube® 16x90mm depyrogenated glass tube | p/n TB16C
References
- Fungitell® Instructions for use https://www.fungitell.com/fungitell_assay#Kit_ifu
- Waters Application Note 720008040EN - Use of the Andrew+™ Robot and OneLab™ Automated Liquid Handling Platform for the Fungitell® Glucan Detection Assay (https://www.waters.com/content/dam/waters/en/app-notes/2023/720008040/720008040-en.pdf)
- Tanaka, S., Aketagawa, J., Takahashi, S., Tsumuraya, Y., and Hashimoto, Y. 1991. Activation of a Limulus coagulation factor G by (1 3)-ß-D-Glucans. Carbohydrate Res. 218:167-174.
- Barsanti, L., Passarelli, V., Evangelista, V., Frassanito, A. M., & Gualtieri, P. (2011). Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Natural product reports, 28(3), 457-466.
- Muta, T. (2006). Molecular basis for invertebrate innate immune recognition of (1→ 3)-β-D-glucan as a pathogen-associated molecular pattern. Current pharmaceutical design, 12(32), 4155-4161.
- Odabasi, Z., Paetznick, V., Rodriguez, J., Chen, E., McGinnis, M., and Ostrosky-Zeichner, L. 2006. Differences in beta-glucan levels of culture supernatants of a variety of fungi. Medical Mycology 44: 267-272.
- Fungitell® Bulletin v2i1 - 2011 - Beta-Glucan (BG) Kinetics as a Biomarker
- De Pauw, B., Walsh, T.J., Donnelly, J.P. et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer /Invasive Fungal Infections Cooperative Group and the National Institutes of Allergy and Infectious disease Mycosis Study Group (EORTC/MSG) Consensus Group. Clin. Inf. Dis. 46: 1813-1821.
Learn more about Fungitell® Assay from Associates of Cape Cod, Inc. (ACC)
Protocols
Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 