or start from open source methods. Learn more about OneLab softwareUse OneLab
EndoLISA® Detection Assay
This example method provides a freely adjustable framework for measuring the adaptability of the OneLab environment to workflows from different application fields. It helps to understand various nuances of the code-free, universal protocol designer and provides general indications as to the feasibility of a project. Scripts generally require fine adjustment to correct for variables and support specific labware implementation.
Overview
Pyrogens are substances, usually of biological origin, that induce an immune response and fever once introduced in the organism (1). The most commonly studied pyrogen is the lipopolysaccharide (LPS), also known as endotoxin, which is an integral component of the outer membrane of gram-negative bacteria and essential for their viability and virulence (2). The LPS complex consists of lipid A, core oligosaccharide, and O-antigen repeats also called O-specific polysaccharide chain (Figure 1). Lipid A is anchored into the outer membrane while hydrophilic core oligosaccharide and O-antigen repeats are exposed on the surface of bacteria (1). Lipid A is responsible for the pathogenicity of LPS (2, 3). Among the three parts of LPS, the structure of lipid A is more widely conserved in different bacteria than that of core oligosaccharide or O-antigen (2).
Endotoxins are released when the bacteria die and their outer membranes disintegrate (3, 4). These potent and ubiquitous toxic molecules and a major challenge to the biopharmaceutical and medical industries. They are heat-stable and remain intact after sterilization of products and elimination of contaminating gram-negative organisms (3). Once introduced into the human system through contaminated medical devices or medications, they trigger an immune response leading to severe physiological reactions especially fever, inflammation, sepsis, multiple organ failure, and even death. Therefore, reliable endotoxin testing is crucial to ensuring product quality and patient safety. For this, sensitive, accurate and rapid detection of endotoxin must be performed, not only on sterilized end-product drugs or medical devices but also on raw materials and for in-process monitoring (4).
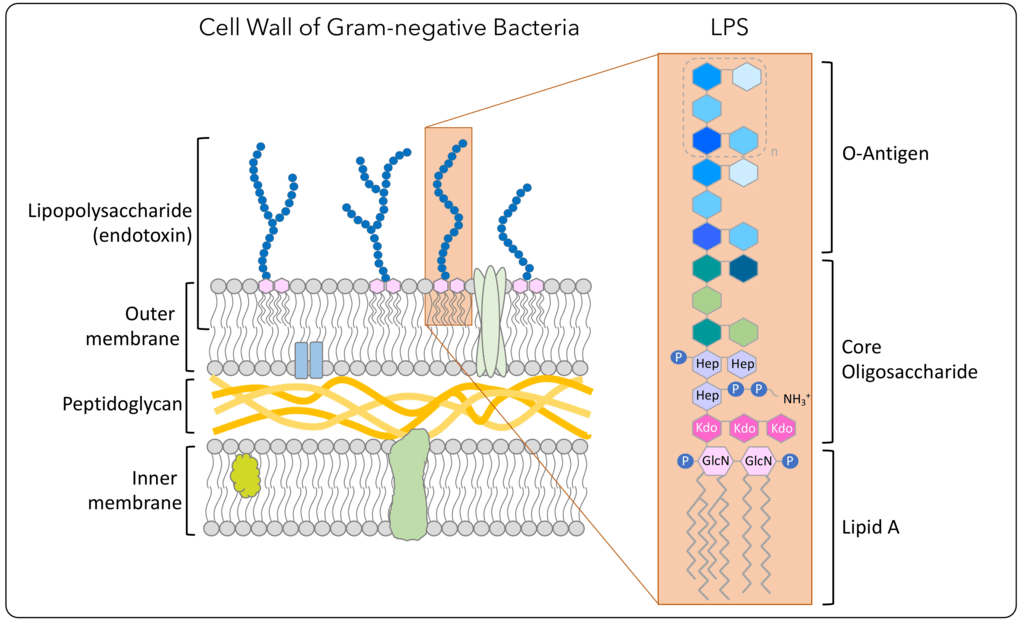
Figure 1: Illustration of the LPS biochemical structure present in the outer membrane of gram-negative bacteria (source: Regarding Endotoxin, AB Biosciences).
Conventional Methods of Endotoxin Detection and Limitations
The Limulus Amoebocyte Lysate (LAL) has been widely used for the detection of bacterial endotoxin in quality control of pharmaceuticals, parenteral drugs, biologics, medical devices, water, and food security as well as for quantitation of endotoxin in clinical settings as diagnostic aid (1, 3). It has gradually replaced the rabbit pyrogen test (RPT), which consisted of injecting the test product into rabbits and monitoring for an increase in body temperature (3). The latter was costly and time-consuming, and the use of living animals has restricted its application for the assessment of a large number of samples.
The LAL assay uses the blood extract of horseshoe crabs, Limulus polyphemus. Its principle of detection is based on the coagulation reaction of LAL induced by endotoxins. The gel-clot assay is the most popular and the simplest LAL-based method used to test the presence or absence of endotoxins (3, 4). The coagulation of LAL is mediated by a protease cascade consisting of three pro-enzymes Factor C, Factor B, and proclotting enzyme, and the protein coagulogen (Figure 2). In presence of endotoxin, zymogen (precursor of a protease) Factor C is activated, which in turn activates Factor B. The active form of Factor B converts the proclotting enzyme into a clotting enzyme, which cleaves the coagulogen to yield a gel clot (3). Other LAL-based methods such as colorimetric, chromogenic, and turbidometric assays were progressively introduced enabling more quantitative measurement of endotoxin concentration (4).
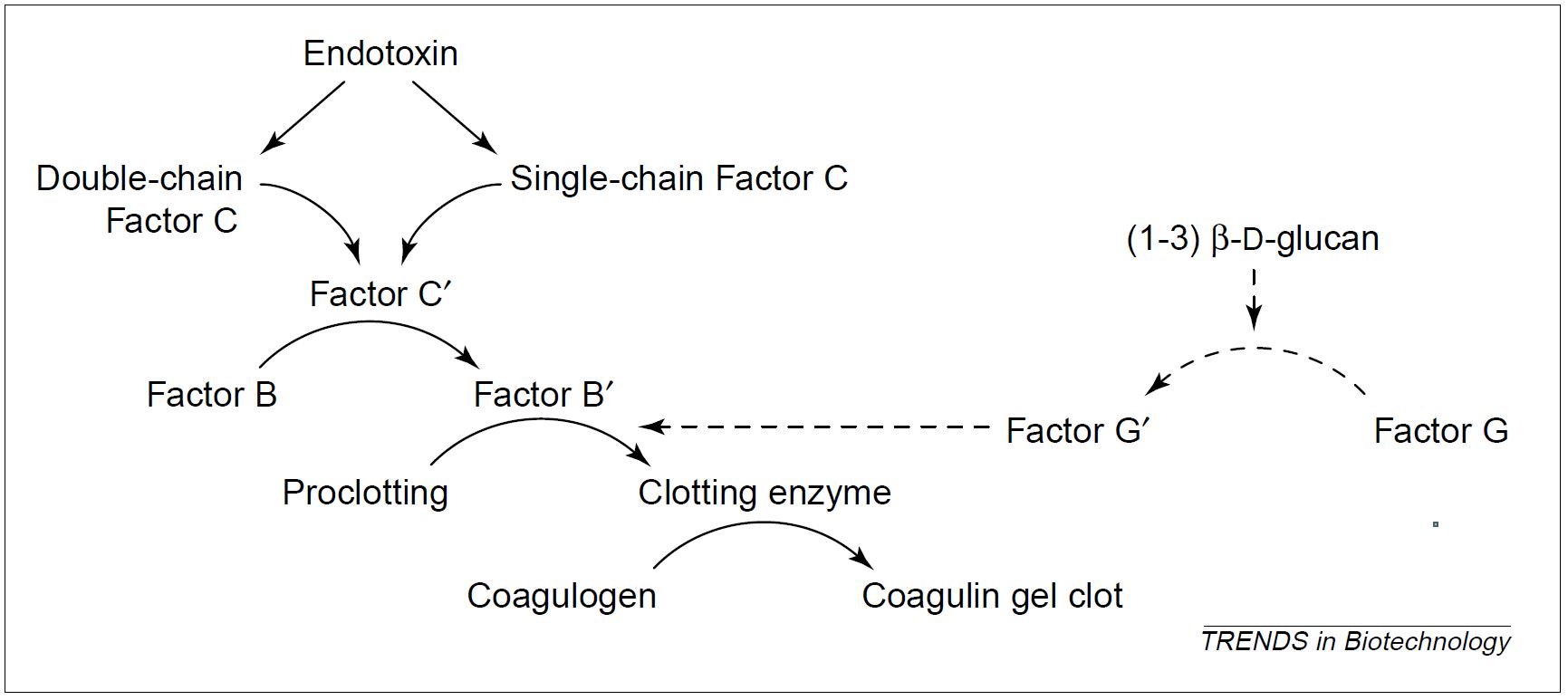
Figure 2: The coagulation cascade reaction in the horseshoe crab amoebocyte lysate. Endotoxin activates both forms of Factor C (1). The pathway with dotted arrows is an endotoxin-independent alternate mechanism, in which Factor G is activated by (1-3) β-D-glucan.
Although the LAL test has been described as a reliable method for detecting endotoxin, it suffers from several defects including:
- Substances present in the test sample and often introduced during the preparation procedure could interfere with any of the steps in the coagulation cascade, thus altering the measurement sensitivity and affecting the final result. For instance, the presence of the chelating agent EDTA at a high concentration in the sample can inhibit the endotoxin-induced coagulation reaction, indicating that divalent cations are essential for the integrity of the LAL assay (1). Therefore, dilution of the sample is required at the expense of sensitivity to lower the concentrations of interfering substances.
- Although LAL is specific to endotoxin, many non-pyrogenic components can disturb (inhibit or stimulate) the assay reaction. When applied to a cell lysate, the LAL results are susceptible to the effect of other proteins. For instance, a LAL cross-reactivity is observed with (1-3) β-D-glucan, a non-pyrogenic fungal contaminant (Figure 2). The latter can activate Factor G, which induces a coagulation cascade, yielding a false-positive result in the LAL assay (3).
- Lot-to-lot fluctuation in the sensitivity of commercial LAL reagent to endotoxin (1).
- The LAL test relies on the blood cells of horseshoe crabs, which are considered to be vulnerable and critically endangered (1).
These limitations and the difficulties to meet the growing demand for LAL-based reagents in the biopharmaceutical industry have prompted an urgent need to find a reliable alternative to the LAL source and LAL testing methods.
Recombinant Fc as an Endotoxin Biosensor and Advantages
The enzymatic components of the coagulation cascade of the Limulus amoebocyte lysate (LAL) have been well characterized (Figures 2 and 3), with zymogen Factor C being the principal receptor of this proteolytic cascade (1). This endotoxin-sensitive serine protease acts as a natural biosensor for endotoxin detection. This essential role of Factor C in the coagulation reaction led to the cloning and expression of a genetically engineered recombinant Factor C (rFC). The biologically functional rFC represents a perpetual, economical, and more ethical alternative to LAL for the detection of bacterial endotoxin (1). In a typical rFC fluorometric endotoxin assay, the zymogen rFC is catalytically activated by trace levels of endotoxin present in a sample. The endotoxin-activated rFC hydrolyses a synthetic fluorogenic substrate to yield a quantifiable fluorescent product (Figure 3), which indicates the level of endotoxin in the test sample (1).
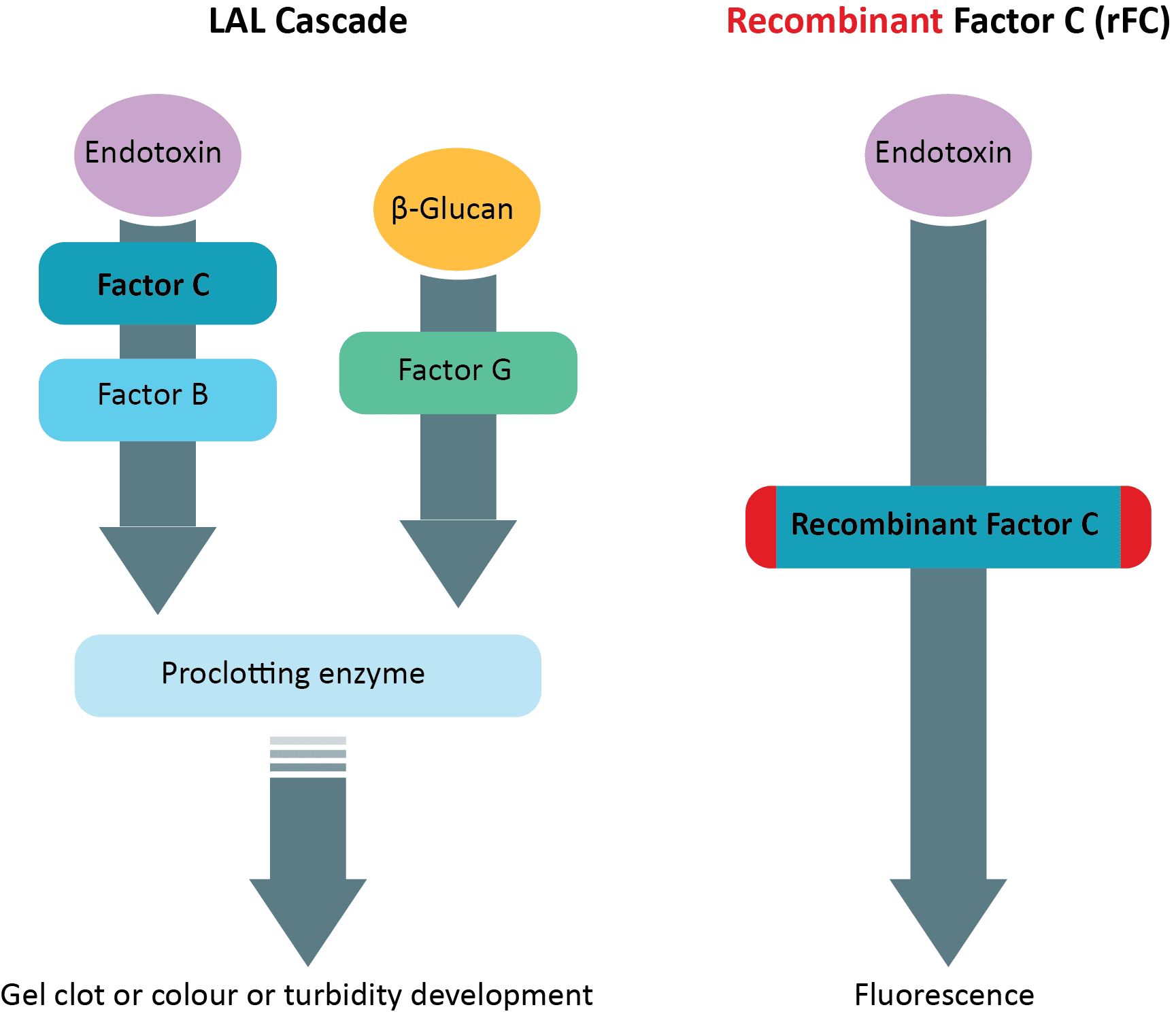
Figure 3: Reaction cascades in Limulus Amoebocyte Lysate (LAL) and recombinant Factor C (rFC) fluorometric endotoxin assay.
The recombinant Factor C shows an enhanced sensitivity of detection and a lower background reading, enabling more accurate quantitation of endotoxin (1). Because rFC is enzymatically activated by endotoxin, the response is specific, showing the level of endotoxin in a sample without cross-activity with (1-3) β-D-glucan (Figure 3). Therefore, rFC is a sustainable and secure source for the development of reliable and fast endotoxin tests.
EndoLISA, a Reliable Endotoxin Detection Assay based on ELISA-Technology and rFC
EndoLISA, developed by Hyglos GmbH (a bioMérieux company), is an ELISA (enzyme-linked immunosorbent assay)-like quantitative fluorescence end-point assay designed for highly specific and robust endotoxin testing in pharmaceutical drugs, research samples, and medical devices. This microplate-based assay uses a bacteriophage-derived receptor protein as a solid phase, coated onto microplate wells to specifically bind LPS and a recombinant Horseshoe Crab Factor C-based reagent for detection of immobilized LPS (5). The phage-derived receptor protein exhibits high affinity and high specificity for the conserved region of LPS (carbohydrate structure of lipid A and inner core oligosaccharide).
When a sample is applied to a microtiter well, LPS molecules selectively bind to the endotoxin-specific phage binding protein on the surface of the pre-coated plate (Figure 4). The sample matrix is then efficiently washed off, thereby removing potentially interfering components prior to the enzymatic detection with rFC (5). The assay reagent, containing equal parts of zymogen rFC and fluorogenic substrate in assay buffer, is then added to the well (Figure 4). Subsequently, rFC is activated by the immobilized LPS, which finally reacts with the fluorogenic substrate, generating a fluorescence signal (5). The latter is detected using a fluorescence reader. The amount of endotoxin in the test sample is measured by extrapolation using a standard curve (5).
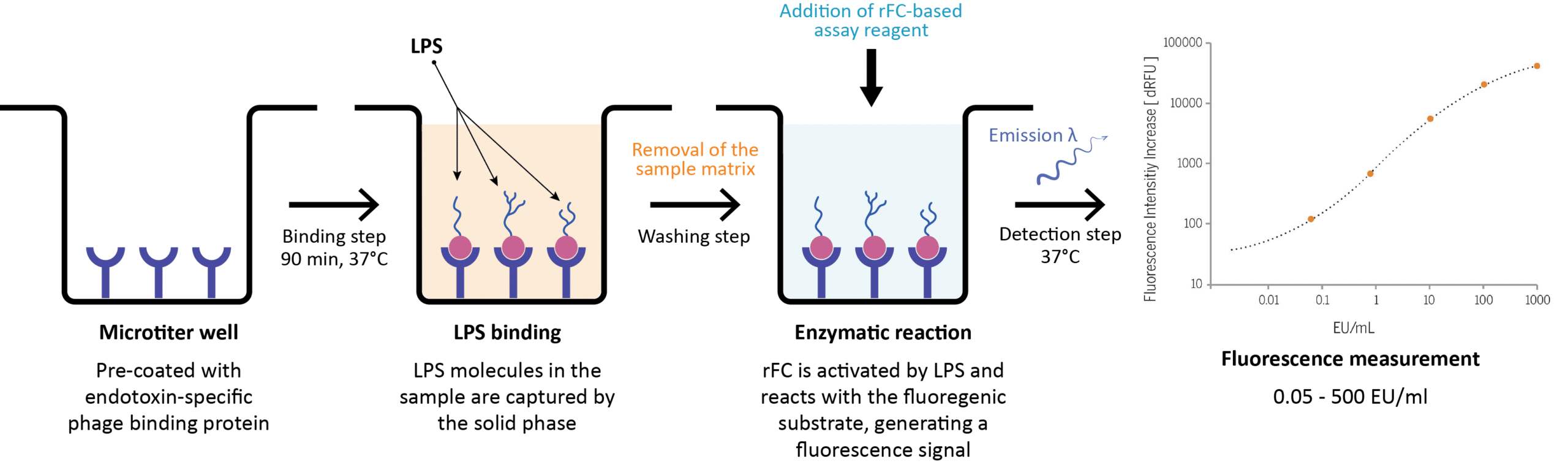
Figure 4: Schematic overview of the EndoLISA® test workflow for endotoxin detection. The graph represents the standard curve of the EndoLISA® assay using a non-linear 4-parameter logistic regression model. Concentrations of the CSE (Control Standard Endotoxin) are plotted against the relative fluorescence signal. EU, endotoxin unit.
EndoLISA has a broad detection range, from 0.05 EU/mL to 500 EU/mL (5) at pH 4 -9. The specified sensitivity limit is 0.05 EU/mL. Because of the elimination of interfering substances by washing, EndoLISA exhibits clear advantages over the conventional LAL methods including no false-positive results induced by β-glucan or other proteases or phospholipids, fewer false-negative results caused by inhibitory components of the test sample, and a lower rate of invalid results necessitating re-testing (5). It has been shown that in the EndoLISA test, sample constituents have a significantly less negative impact than for the LAL assay, allowing precise quantification of endotoxin content in complex pharmaceutical, biological and environmental samples with fewer matrix effects and without the need for extensive dilution (5). With its unique built-in sample preparation and selectivity, endotoxin detection using EndoLISA has gained in robustness and sensitivity and demonstrates excellent lot-to-lot reproducibility.
Automation of EndoLISA® and Method Standardization
ELISA is a well-established technique in biochemical analysis and diagnostics. Its workflow is generally amenable to automation because it involves repetitive and error-prone steps. Being based on ELISA and using a recombinant Factor C, EndoLISA is a good candidate for automation and a suitable method for high-throughput testing in contrast to conventional LAL methods. Andrew Alliance automation solutions facilitate sample and standard preparation and help to minimize variability between assays, delivering better reproducibility as well as a lower rate of invalid results. On the other hand, OneLab contributes to the standardization of the assay especially in controlled environments, enabling easier and more consistent protocol development and validation as well as traceability.
(1) Ding, Jeak L., and Bow Ho. “A New Era in Pyrogen Testing.” Trends in Biotechnology 19, no. 8 (August 1, 2001): 277–81.
(2) Wang, Xiaoyuan, and Peter J. Quinn. “Endotoxins: Lipopolysaccharides of Gram-Negative Bacteria.” Sub-Cellular Biochemistry 53 (2010): 3–25.
(3) Su, Wenqiong, and Xianting Ding. “Methods of Endotoxin Detection.” Journal of Laboratory Automation 20, no. 4 (August 2015): 354–64.
(4) PharmTech. “Comparing Endotoxin Detection Methods.”
(5) Grallert, H., Leopoldseder, S., Schuett, M., Kurze, P., & Buchberger, B. (2011). EndoLISA ®: A novel and reliable method for endotoxin detection. Nature Methods, 8(10), iii–v.
Protocols

Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 