or start from open source methods. Learn more about OneLab softwareUse OneLab
Automation of PFAS Samples in Milk Matrices using SPE

This basic method provides the core methodology for translating a workflow into OneLab-executable script(s) as an attempt to fully or semi-automate a specific procedure. It demonstrates the benefits of automation and highlights OneLab capabilities and best practices to promote solution adoption, helping transition from manual to a more automated approach. It can be used alone or serves as a building block for a more complex workflow and is easily adaptable to users' requirements.
Overview
Per- and Polyfluoroalkyl Substances (PFAS) are a group of man-made chemicals that have multiple fluorine atoms attached to an alkyl chain. Due to its unique properties such as high heat and chemical stability, ability to reduce surface tension of aqueous solutions, and surfactant and oleophobic nature, PFAS are used in many areas for various applications such as packaging, surfactants, and firefighting fluids. However, studies have shown potential links between PFAS exposure and detrimental health effects on humans and animals (1). As such, there is a pressing need to reduce the usage and prevalence of PFAS in all materials, and one important area is to reduce PFAS in food. As PFAS compounds are resistant to degradation and bioaccumulate in the tissues of mammals (2), they can be transferred from food products to humans through consumption. The European Food Safety Authority (EFSA) predicts food to be a predominant channel for PFAS exposure, and regulatory bodies around the world have been placing increasingly stringent regulations on the testing and monitoring of PFAS in food (3). Milk, being the staple food product for toddlers and children, would affect them disproportionately compared to adults, and as such, there is a need to monitor PFAS levels in milk matrices.
To quantify PFAS, a calibration curve is to be built. The EURL POP guidance document for PFAS in food and feed indicates that the range of the calibration curve should span between the limit of quantification (LOQ) to around 5 to 10 µg/kg, and at least five calibration concentrations are required (4). In this protocol, a calibration curve is built from 0.00125 - 5 µg/kg (equivalent to 0.00025 – 1 µg/kg in actual sample) spanning 4000-fold range.
Milk is a complex food matrix, and to analyze trace levels of PFAS, a solid-phase extraction (SPE) protocol using Oasis WAX cartridges can be used to improve extraction recovery and obtain samples with minimized matrix interference. Oasis WAX is a mixed-mode reverse phase-weak anionic exchange polymer that can bind to PFAS that are hydrophobic with anionic side groups. Milk matrices are washed away during the wash step, and finally, PFAS is eluted with basic organic solvents.
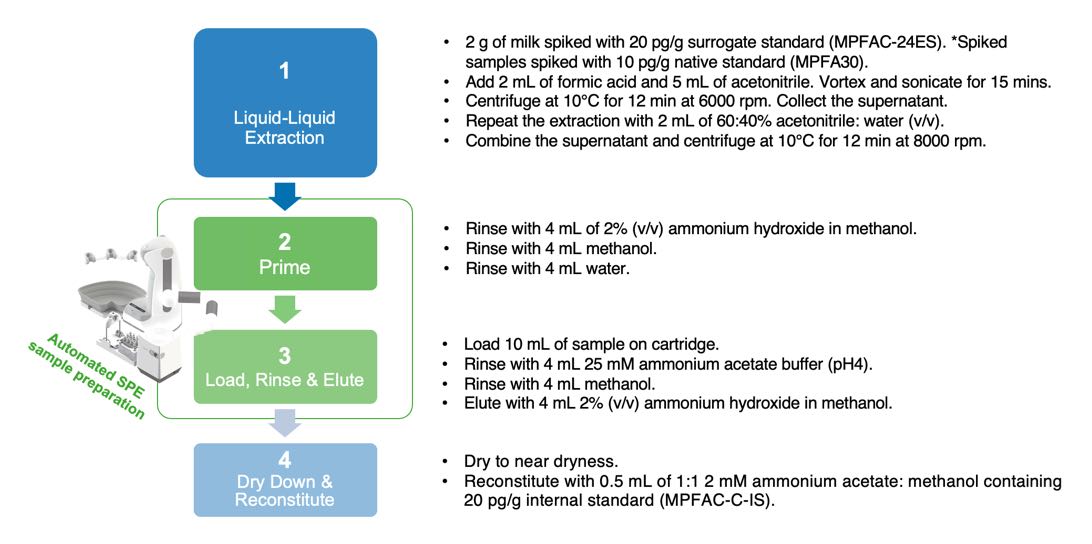
Figure 1: Sample preparation workflow for PFAS in milk using OASIS GCB/WAX
Preparing milk samples for analysis using an LC-MS/MS involves a 4-part workflow requiring many pipetting tasks and is a time-consuming process. It is, therefore, desirable to automate as many of the parts of the workflow to improve throughput and reduce human experimental errors. The protocol shown here automates the priming of Oasis GCB/WAX cartridges, loading, rinsing, and eluting of the sample, thus improving experimental efficiency while reducing errors.
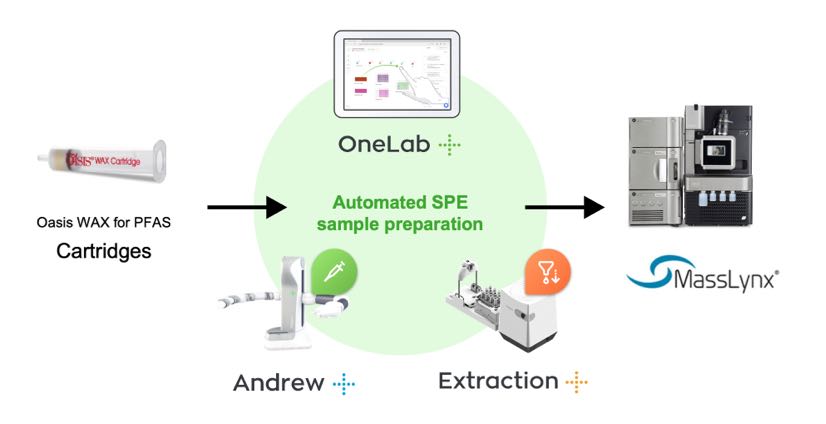
Figure 2: Graphical illustration of the automated SPE sample extraction and LC-MS analysis workflow using the Oasis GCB/WAX for PFAS 6 cc cartridges on the Andrew+ Pipetting Robot configured with the Extraction+ Connected Device.
PFAS contamination from the setup is minimized due to several reasons. The method contains a methanol washing step for pipette tips to ensure they are PFAS-free. Secondly, there is no contact between the Oasis GCB/WAX cartridges and the collection vials, minimizing environmental contamination from the exterior of the cartridges. Finally, there are minimal human actions taken during the protocol, making PFAS extractions accessible to a wider range of technical skill levels.
This protocol makes use of a script within ‘Guidelines’ to wash the inside of the sample tubes to ensure that PFAS are recovered and not adhered to the walls of the sample tube. By specifying 4 points along the inside of the sample tubes, the Andrew+ dispenses solvent at the 4 points, allowing wash solvents to run down the sides of the tubes to collect as much PFAS as possible. Thereafter, a mixing step is introduced to allow wash solvents to be thoroughly agitated in the sample tube, and the wash solvents are then applied to the Oasis GCB/WAX cartridge to be pulled through.
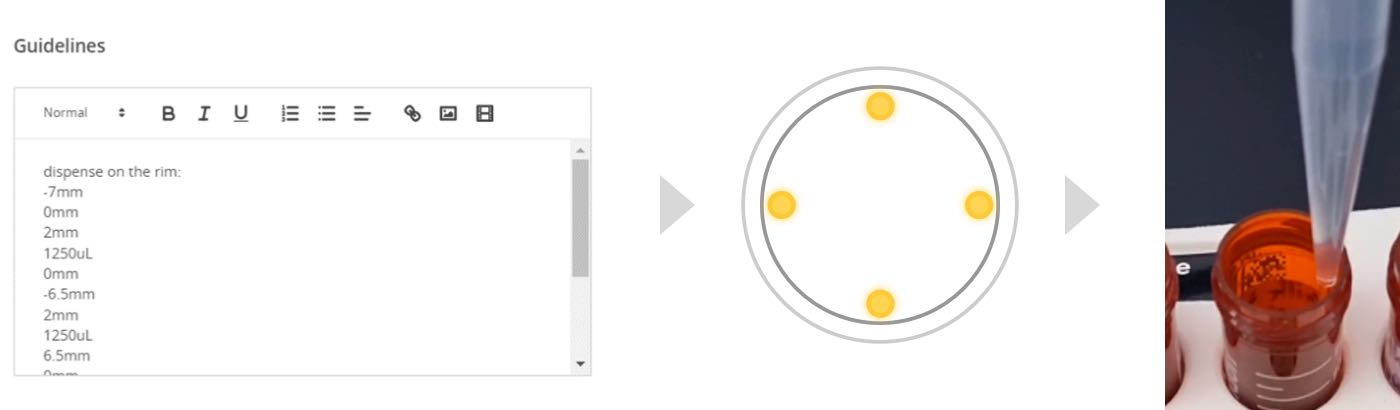
Figure 3: Andrew+ Guidelines to dispense along the rim (left), the 4 positions of solvent dispensing (center) and dispensing along the rim (right).
Assay notes
This procedure is designed as a general protocol and may need to be adjusted to suit individual sample needs or quantities. Sample preparation steps need to be performed before placing the samples into the sample vials. Likewise, drying down and reconstitution need to occur after the protocol finishes, before LC-MS analysis.
Protocol 1: Calibrators for Analysis of PFAS in Milk
Considerations:
The solution in position A1 of the 48x rack is labeled “Native A.” It is made up of 100 µg/kg of PFAC30PAR, L-PFUdS, L-PFDoS, and L-PFTrDS. If required, this solution will need to be manually made up.
The solution in position B1 of the 48x rack is labeled “Iso Std A.” It is made up of 2 µg/kg of C-IS, 24ES, and M3 HFPO-DA. If required, this solution will need to be manually made up.
Figure 4: Samples and required solutions at the start of calibrator preparation

Figure 5: Andrew+ OneLab deck set up for the automated “Calibration Curve for PFAS in Milk” assay. Domino/Device locations on Andrew+ deck: [1] Tip insertion system domino with 50-1000 µl Optifit tips, [2] Tip insertion system domino with 10-300 µl Optifit tips, [3] Tip insertion system domino with 5-120 µl Optifit tips, [4] 2 mL HPLC Vials Domino, [5] 50mL conical Centrifuge Tube Domino, [6] 250mL Duran Bottle Domino
Protocol 2: Preparation of PFAS samples in Milk
Considerations:
This procedure is designed as a general protocol and may need to be adjusted to suit individual sample needs or quantities. Sample preparation steps need to be performed before placing the samples into the sample vials. Likewise, drying down and reconstitution must occur after the protocol finishes before LC-MS analysis.
Edits can include 250mL Duran bottles as waste collection containers instead of 50 ml conical centrifuge tubes.
‘Guidelines’ are scripted to allow Andrew+ to wash the sample tubes' walls with the stated solutions. While the values defined as the edges of the tubes are designed to be universal, small differences may occur per laboratory setup. This protocol may need to be optimized to define the best distance for the pipette to elute solutions along the rim of the sample vials.
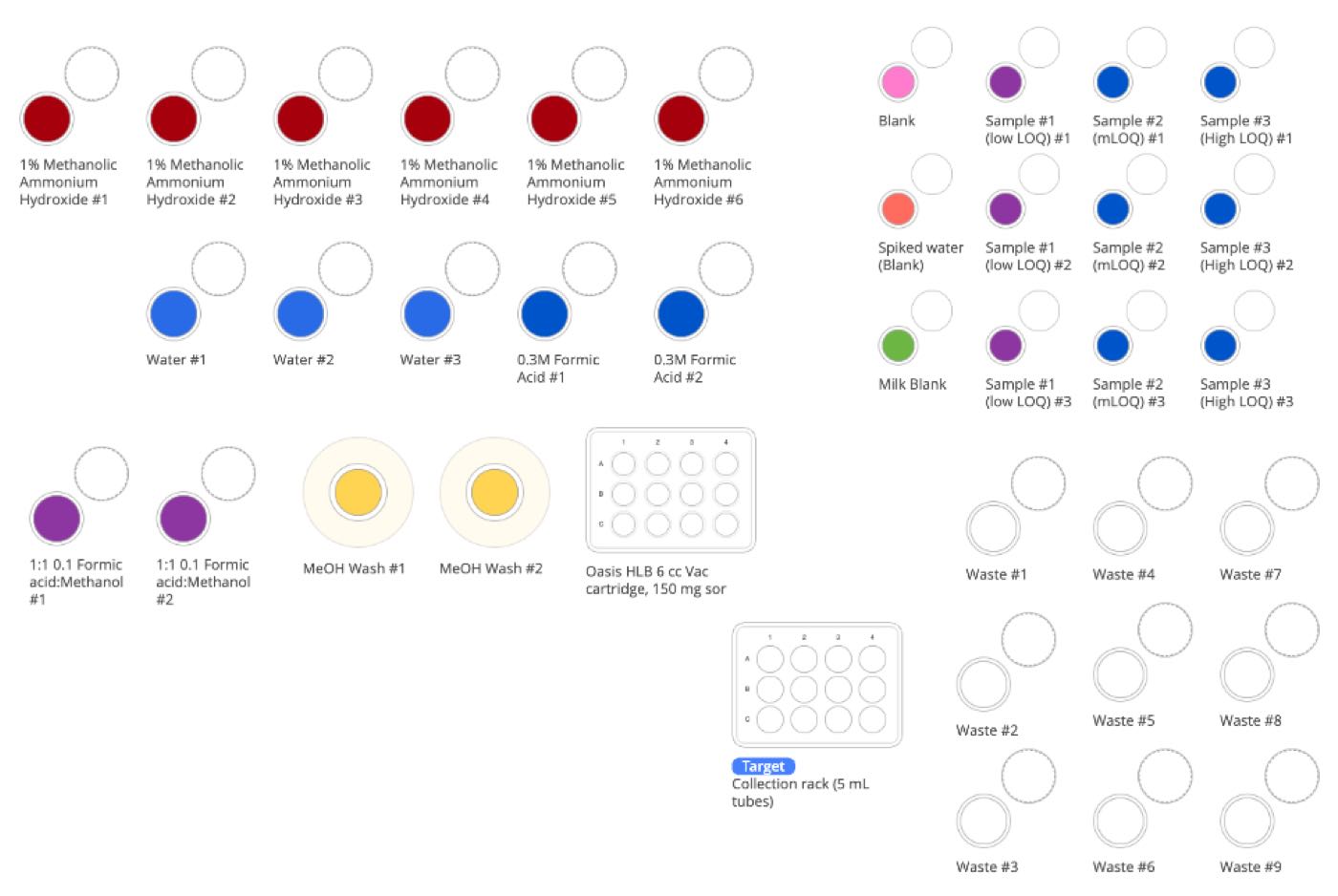
Figure 6: Samples and required solutions at the start of PFAS sample preparation
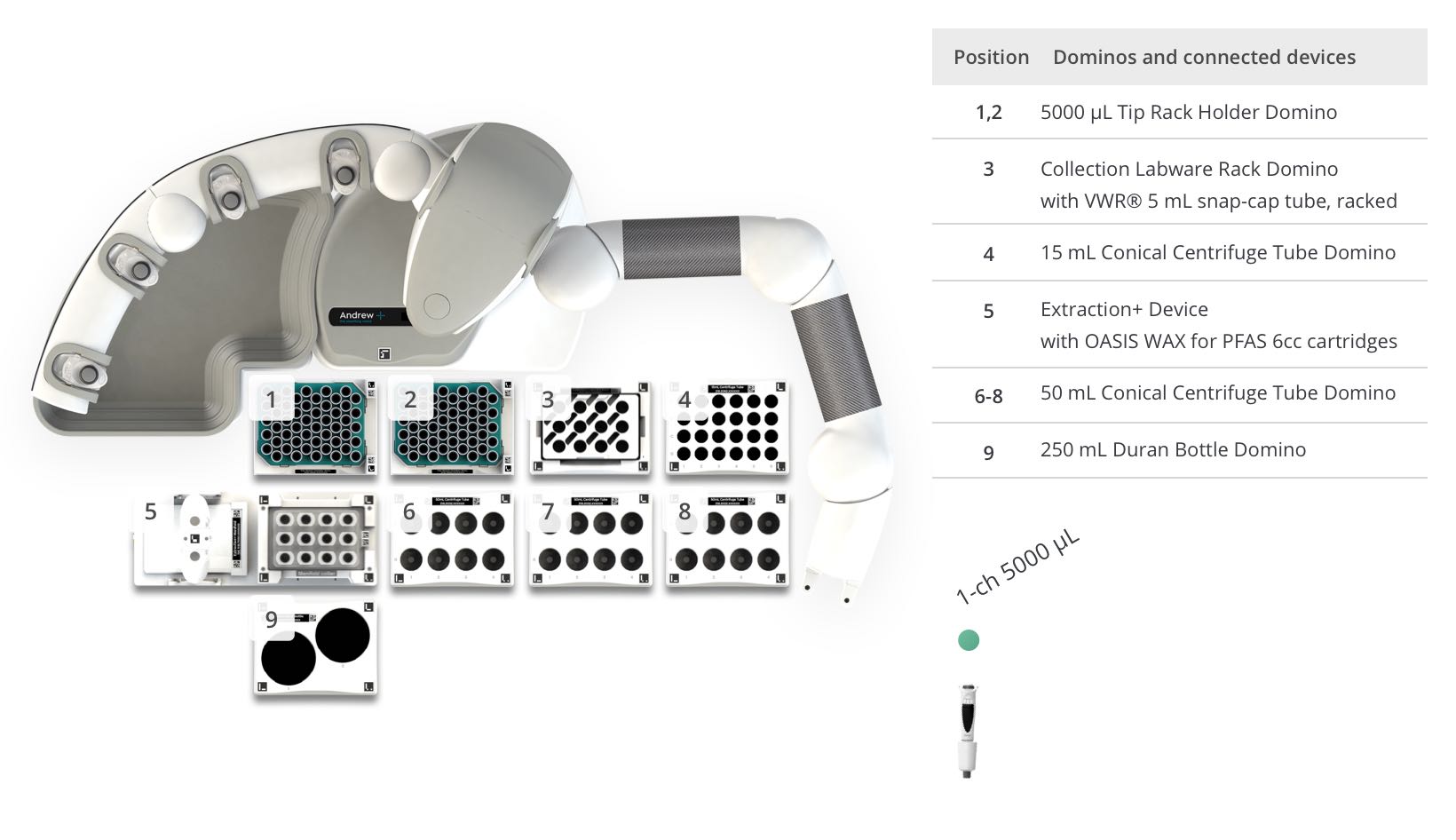
Figure 7: Andrew+ OneLab deck set up for the automated “Preparation of PFAS samples in Milk” assay. Domino/Device locations on Andrew+ deck: [1 - 2] 5ml Tip Rack Holder Domino, [3] Collection Labware Rack Domino with Eppendorf® 5 mL snap-cap tube, racked, [4] 15ml Conical Centrifuge Tube Domino, [5] Extraction+ Device with Oasis WAX 6cc cartridges, [6 - 8] 50ml Conical Centrifuge Tube Domino, [9] 250ml Duran Bottle Domino
Protocols specifications
Calibration Curve for PFAS in Milk:
- Estimated time of execution: 46m 6s
- Hands-on time: 0s
- Tip consumption:
- 30× 100-5000 μL tips
- 25× 10-300 µL tips
- 1× 50-1000 µL tips
Automated PFAS Testing in Milk using Oasis WAX for PFAS - 12 samples:
- Estimated time of execution: 2h 04m 35s
- Hands-on time: 0s
- Tip consumption:
- 89× 100-5000 μL tips
Ordering information
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
- Andrew+ Pipetting Robot
- OneLab software
- 2× 5000 µL Tip Rack Holder Domino | p/n 186009599
- 15ml Conical Centrifuge Tube Domino | p/n 186010087
- 3× 50ml Conical centrifuge Tube Domino | p/n 186009614
- 250mL Duran Bottle Domino | p/n 186010193
- 2mL HPLC Vial Rack Domino | p/n 186010091
- Extraction+ Base Kit with Plate Gripper | p/n 176005201
- Extraction+ 6cc Cartridge Kit including 1x Collection Labware Rack Domino | p/n 176005206
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 120 μL | p/n 186009765
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 5000 μL | p/n 186009608
Recommended consumables
- Waters Polypropylene 12 x 32 mm Screw Neck Vial, with Cap and Preslit PTFE/Silicone Septum, 700 µL Volume, 100/pk | p/n 186005221
- Waters 48-position Vial Holder | p/n 405000562
- Sartorius, Optifit Tips, 0.5-200 µL | p/n 700013295
- Sartorius, Optifit Tips, 5-350 μL | p/n 700013297
- Sartorius, Optifit Tips, 10-1000 μL | p/n 700013298
- Sartorius, Optifit Tips, 100-5000 μL | p/n 700013291
- 2× DURAN® 250 mL clear glass laboratory bottle | p/n 218013651
- 12× Eppendorf Tubes® 5 mL snap-cap centrifuge tube | p/n 0030119401
- 22× VWR® 50 mL conical centrifuge tube | p/n 525-0610
- 12× Eppendorf Tubes® 15mL conical centrifuge tubes | p/n 0030122194
- Oasis WAX for PFAS Analysis 6 cc Vac Cartridge, 50 mg GCB, 200 mg WAX, 60 µM WAX Particle Size, 30/pk | p/n 186011112
- or Oasis WAX for PFAS Analysis 6 cc Vac Cartridge, 50 mg GCB, 200 mg WAX, 60 µM WAX Particle Size, 300/pk | p/n 186011113
References
- Temkin, A. M., Hocevar, B. A., Andrews, D. Q., Naidenko, O. V., & Kamendulis, L. M. (2020). Application of the key characteristics of carcinogens to per and polyfluoroalkyl substances. International Journal of Environmental Research and Public Health, 17(5), 1668. https://doi.org/10.3390/ijerph17051668
- Cousins, I. T., DeWitt, J. C., Glüge, J., Goldenman, G., Herzke, D., Lohmann, R., Ng, C. A., Scheringer, M., & Wang, Z. (2020). The high persistence of pfas is sufficient for their management as a chemical class. Environmental Science: Processes & Impacts, 22(12), 2307–2312. https://doi.org/10.1039/d0em00355g
- PFAS in food: EFSA assesses risks and sets tolerable intake. European Food Safety Authority. (n.d.). https://www.efsa.europa.eu/en/news/pfas-food-efsa-assesses-risks-and-sets-tolerable-intake#:~:text=The%20threshold%20%E2%80%93%20a %20group%20tolerable,of%20these%20substances%20in%20food
- 4. EURL for halogenated POPs in feed and food (2022): Guidance Document on Analytical Parameters for the Determination of Per- and Polyfluoroalkyl Substances (PFAS) in Food and Feed, version 1.2, 11 May 2022. https://eurl-pops.eu/core-working-groups#_pfas
Protocols
Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 