or start from open source methods. Learn more about OneLab softwareUse OneLab
Automation of PFAS Extraction in Firefighting Foam using SPE with Oasis WAX/GCB cartridges
This basic method provides the core methodology for translating a workflow into OneLab-executable script(s) as an attempt to fully or semi-automate a specific procedure. It demonstrates the benefits of automation and highlights OneLab capabilities and best practices to promote solution adoption, helping transition from manual to a more automated approach. It can be used alone or serves as a building block for a more complex workflow and is easily adaptable to users' requirements.
Overview
Per- and Polyfluoroalkyl Substances (PFAS) are synthetic chemicals characterized by an alkyl chain with multiple fluorine atoms. Because of its high heat stability and ability to reduce surface tension of aqueous solutions, PFAS can form a film layer at the fuel-air interface to deprive fire of oxygen and suppress fuel flammable vapors. Therefore, PFAS has been used a major component in Class B firefighting fluids (FFF) to fight flammable liquids, and can reach up to 6% (v/v) [1].
PFAS has been shown to be carcinogenic and endocrine disrupting [2]. In addition, PFAS is resistant to degradation and bio-accumulative, therefore exposure to FFF with high concentration of PFAS can have negative effects on health. There are increasing regulations of PFAS in FFF. In the US, the National Defense Authorization Act requires the Department of Defense to stop using PFAS-based firefighting foams by 2024 [3]; in the European Union, the European Chemicals Agency has brought forward a proposal to restrict all PFAs in firefighting foams [4]; in Singapore, the National Environment Agency is regulating the phasing out of fire-fighting foams containing PFOA (> 25 ppb), PFOS (> 10 000 ppb) and PFHxS (> 100 ppb) by 1 Jan 2026 [5].
The US Department of Defense has developed an analytical method (DoD AFFF01) to identify and quantify PFAS in aqueous film-forming foams (AFFF) by diluting the foam sample followed by solid-phase extraction to with Oasis WAX cartridges to reduce matrix and to extract PFAS for LC-MS/MS analysis [6]. Oasis WAX is a mixed-mode reverse phase-weak anionic exchange polymer that can bind to PFAS that are hydrophobic with anionic side groups. After SPE, samples are further cleaned up with graphitized carbon black (GCB), which removes interfering matrix contaminants through polar sites. In this protocol, the US DoD AFFF01 method is adapted here for usage with the Andrew+ automation system with bilayer Oasis WAX/GCB cartridge, which includes both the WAX later and a GCB layer after. This reduces the number of steps needed to run the protocol while still provided the carbon cleanup after the main extraction.
This OneLab protocol also includes making of solution standards for building of a calibration curve. In this protocol, 9 solution standards from a concentration range of 0.05 – 100 ng/mL (equivalent to 12.5 – 2500 ng/ml in FFF matrix) spanning 2000-fold range were made with the Andrew+ pipetting robot.
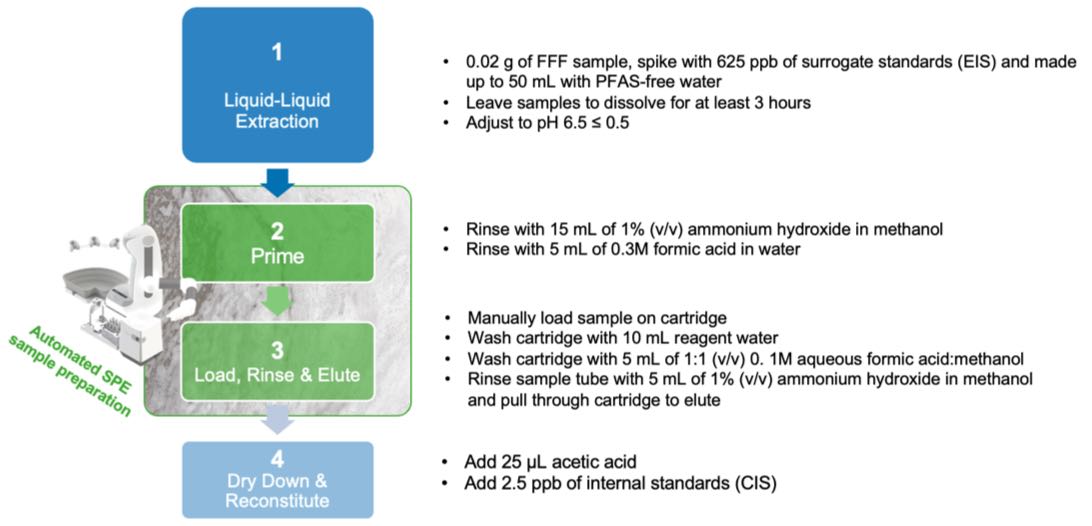
Figure 1: Sample preparation workflow for PFAS in AFFF using OASIS GCB/WAX
Preparing AFFF samples for analysis with LC-MS/MS involves a complex, four-step workflow that requires extensive pipetting and is time-consuming. Automating as much of this process enhances throughput and minimize human error. The protocol presented here automates the priming of Oasis WAX/GCB cartridges, as well as the loading, rinsing, and eluting of samples, thereby improving experimental efficiency and reducing errors.
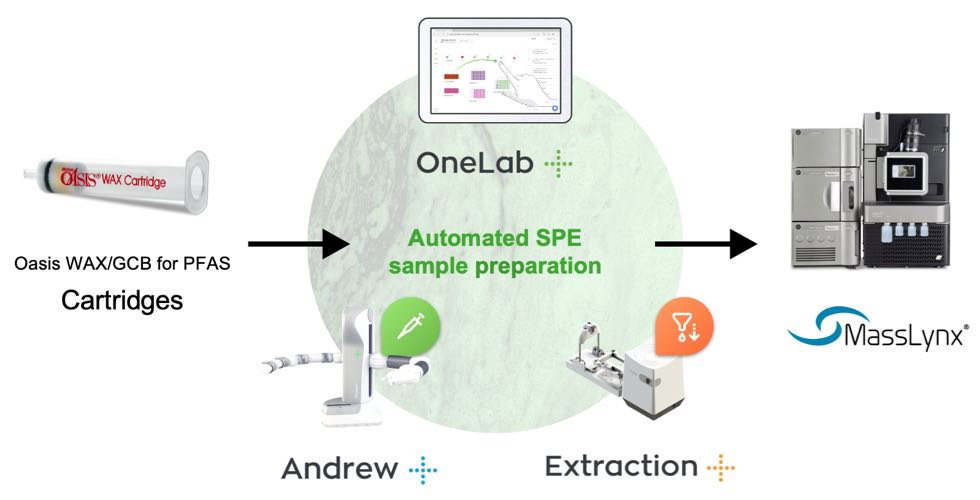
Figure 2: Graphical illustration of the automated SPE sample extraction and LC-MS analysis workflow using the Oasis GCB/WAX for PFAS 6 cc cartridges on the Andrew+ Pipetting Robot configured with the Extraction+ Connected Device.
Assay notes
Protocol 1: Calibrators for Analysis of PFAS in FFF
Considerations:
The solution in position A1, B1, B2, and B3 of the 48x rack are PFAC30PAR, MPFAC-C-IS, MPFAC-24ES and M3-HFPODA (Wellington Laboratories).
Other stock solutions can be used, but dilutions will be required prior to starting this protocol so that the initial concentration in each vial remains at the specified amounts.
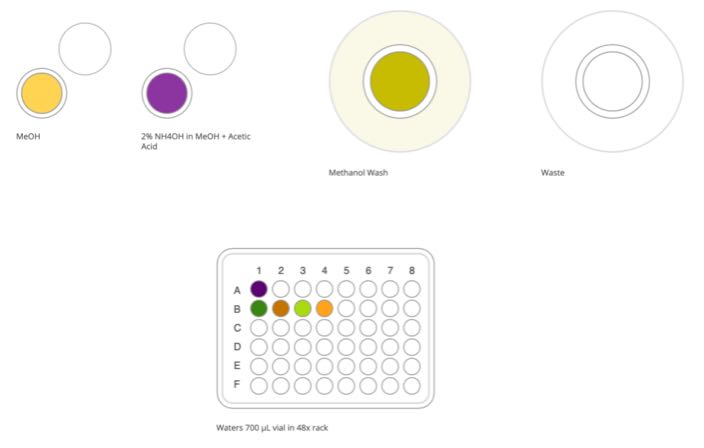
Figure 3: Samples and required solutions at the start of calibrator preparation

Figure 4: Andrew+ OneLab deck set up for the automated “Calibration Curve for PFAS in FFF” assay. Domino/Device locations on Andrew+ deck: [1] Tip insertion system domino with 50-1000 µL Optifit tips, [2] Tip insertion system domino with 10-300 µL Optifit tips, [3] Tip insertion system domino with 0.1-10 µL Optifit tips, [4] 2 mL HPLC Vials Domino, [5] 15 mL conical Centrifuge Tube Domino, [6] 250 mL Duran Bottle Domino
Protocol 2: Preparation of PFAS samples in FFF
Considerations:
his procedure is designed as a general protocol and may need to be adjusted to suit individual sample needs or quantities. Sample preparation steps need to be performed before placing the samples into the sample vials. Likewise, 25 µL of acetic acid needs to be added to each sample after the protocol finishes prior to LC-MS analysis.
During sample loading, the protocol turns on the vacuum for an extended period of time, allowing the user to manually pour pre-prepared sample into cartridges. Matrix build-up from sample loading can hinder flow through the Oasis GCB/WAX cartridge and can become stuck. Furthermore, the constant vacuum and sample pouring required means the solution in the cartridge can be completely pulled through before more can be added to the cartridge. The pressure gradient programmed during these steps is such that the pressure acting on the cartridges consistently increases momentarily. This allows the sample pull-through to overcome the increased resistance from the matrix buildup, as well as to kickstart any pull through of fresher solvent.
During the final step, the User Action states, “Rinse sample tubes with 1% methanolic NH4OH and place in cartridge”. It is recommended that at this step, the user adds 5mL of 1% methanolic ammonium hydroxide into the sample tubes and swirl to ensure any PFAS along the walls of the sample tube are dissolved. The user will then click ‘Next’, and the Andrew+ robot will bring the Extraction+ manifold over the collection vials and the vacuum profile is started. Thereafter, the user will manually pour each elution solvent from the sample tubes into their respective cartridges.
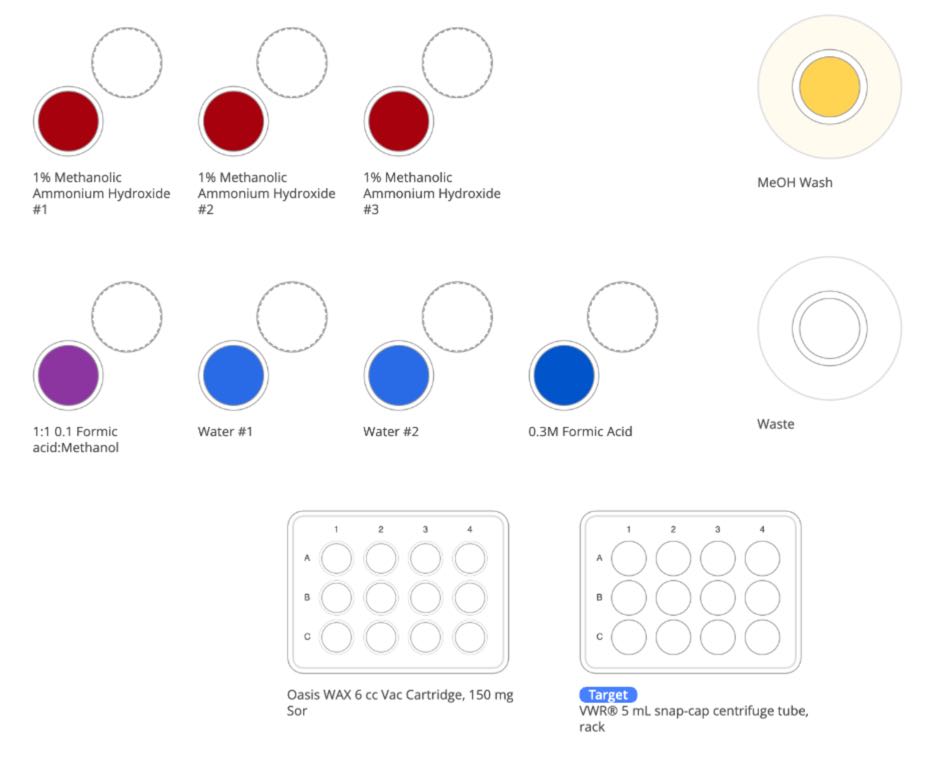
Figure 5: Samples and required solutions at the start of PFAS sample preparation

Figure 6: Andrew+ OneLab deck set up for the automated “Preparation of FFF using Oasis WAX/GCB for PFAS Analysis” assay. Domino/Device locations on Andrew+ deck: [1] 5 mL Tip Rack Holder Domino, [2] Collection Labware Rack Domino with Eppendorf® 5 mL snap-cap tube, racked, [3] 50ml Conical Centrifuge Tube Domino, [4] 250 mL Duran Bottle Domino, [5] Extraction+ Device with Oasis WAX 6cc cartridges
Protocols specifications
Calibration Curve for PFAS in FFF:
- Estimated time of execution: 37 min
- Hands-on time: 0s
- Tip consumption:
- 19× 0.1 – 10 µL tips
- 25× 10 – 300 µL tips
- 2× 50 – 1000 µL tips
Automated PFAS Testing in FFF:
- Estimated time of execution: 1 h 22 min
- Hands-on time: 4 min
- Tip consumption:
- 5× 100-5000 μL tips
Ordering information
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
- Andrew+ Pipetting Robot
- OneLab software
- 3× Tip Insertion System Domino | p/n 186009612
- 5000 µL Tip Rack Holder Domino | p/n 186009599
- 15ml Conical Centrifuge Tube Domino | p/n 186010087
- 50ml Conical centrifuge Tube Domino | p/n 186009614
- 250mL Duran Bottle Domino | p/n 186010193
- 2mL HPLC Vial Rack Domino | p/n 186010091
- Extraction+ Base Kit with Plate Gripper | p/n 176005201
- Extraction+ 6cc Cartridge Kit including 1x Collection Labware Rack Domino | p/n 176005206
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 10 μL | p/n 186009769
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 1000 μL | p/n 186009766
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 5000 μL | p/n 186009608
Recommended consumables
- Waters Polypropylene 12 x 32 mm Screw Neck Vial, with Cap and Preslit PTFE/Silicone Septum, 700 µL Volume, 100/pk | p/n 186005221 in Waters 48x Rack | p/n 700011047
- Waters 48-position Vial Holder | p/n 405000562
- Sartorius, Optifit Tips, 0.1-10 μL | p/n 700013293
- Sartorius, Optifit Tips, 5-350 μL | p/n 700013297
- Sartorius, Optifit Tips, 10-1000 μL | p/n 700013298
- Sartorius, Optifit Tips, 100-5000 μL | p/n 700013291
- 4× DURAN® 250 mL clear glass laboratory bottle | p/n 218013651
- 9× VWR® 50 mL conical centrifuge tube | p/n 525-0610
- 9× Eppendorf Tubes® 5 mL snap-cap centrifuge tube | p/n 0030119401
- Oasis WAX/GCB for PFAS Analysis 6cc Vac Cartridge, 200 mg WAX, 50 mg GCB, 60 µm WAX Particle Size, 30/pk | p/n 186011110
References
- 1. Young, A.S., Sparer-Fine, E.H., Pickard, H.M., Sunderland, E.M., Peaslee G.F., Allen, J.G (2021) Per- and polyfluoroalkyl substances (PFAS) and total fluorine in fire station dust. J Expo Sci Environ Epidemiol 31, 930–942. https://doi.org/10.1038/s41370-021-00288-7
- Cousins, I. T., DeWitt, J. C., Glüge, J., Goldenman, G., Herzke, D., Lohmann, R., Ng, C. A., Scheringer, M., & Wang, Z. (2020). The high persistence of pfas is sufficient for their management as a chemical class. Environmental Science: Processes & Impacts, 22(12), 2307–2312. https://doi.org/10.1039/d0em00355g
- Mokra, K. (2021) Endocrine Disruptor Potential of Short- and Long-Chain Perfluoroalkyl Substances (PFASs)—A Synthesis of Current Knowledge with Proposal of Molecular Mechanism. Int. J. Mol. Sci. 2021, 22, 2148. https://doi.org/10.3390/ijms22042148
- Office, U. S. G. A. (2024). Firefighting Foam: DOD is Working to Address Challenges to Transitioning to PFAS-Free Alternatives. U.S. GAO-24-107322 https://www.gao.gov/products/gao-24-107322
- ECHA (2022). Proposal to ban ‘forever chemicals’ in firefighting foams throughout the EU. ECHA/NR/22/05. https://echa.europa.eu/-/proposal-to-ban-forever-chemicals-in-firefighting-foams-throughout-the-eu
- National Environment Agency (2024). Phasing out of Fire-fighting foams containing per- and polyfluoroalkyl substances (PFAS) chemicals listed under the Stockholm Convention. NEA/HS/6.6. https://www.nea.gov.sg/docs/default-source/default-document-library/phase-out-of-fire-fighting-foams-containing-pfas-chemicals-listed-under-the-stockholm-convention_15mar24.pdf
- Willey, J.L. (2021) Determination of perfluorooctanoic acid and perfluorooctanesulfonic acid in aqueous film forming foam (AFFF) for demonstration of compliance to MIL-PRF-24385. DoD AFFF01 rev. 1.0. https://www.denix.osd.mil/edqw/denix-files/sites/43/2021/12/DoD-AFFF01-Rev-1.0-dtd-7-Dec-2021-1.pdf
Protocols


Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 