or start from open source methods. Learn more about OneLab softwareUse OneLab
Automated mAb Subunit Screening
This basic method provides the core methodology for translating a workflow into OneLab-executable script(s) as an attempt to fully or semi-automate a specific procedure. It demonstrates the benefits of automation and highlights OneLab capabilities and best practices to promote solution adoption, helping transition from manual to a more automated approach. It can be used alone or serves as a building block for a more complex workflow and is easily adaptable to users' requirements.
Overview
Introduction
Rapid and high-throughput screening of mAbs in the form of subunits can potentially enhance development and improve the overall quality of biopharmaceutical products while reducing manufacturing costs. In general, mAb subunits (23-25 kDa in size) are generated by using FabRICATOR (IdeS) protease to digest antibodies at a specific site below the hinge followed by DTT reduction of the disulfide bonds. Subunits are more homogeneous than the intact mAb and of low enough mass to allow for the acquisition of adequate-quality spectra with more moderately priced MS instruments while still providing considerably shorter analysis time and simplified data interpretation.
Here we present a fully automated workflow for purification and digestion of therapeutic mAbs harvested directly from cell culture media. This method is based on purification using magnetic protein A beads followed by a combined digestion/reduction. An Andrew+ pipetting robot was used to automate the entire sample preparation process which yielded subunits ready for analysis by the Waters BioAccord LC-MS system. Small volumes (20 μL to 100 μL) of the mAb media samples were evaluated for this study either directly (non-purified media) or as Protein A-purified samples for intact, subunit level analyses.
Sample Preparation & Analysis
Using an Andrew+ Pipetting robot, a fully automated workflow was developed for sample preparation (Figure 1). Purification of the mAbs from cell culture media was performed by incubating 100 μL of the sample (with the indicated concentration) with Magne® Protein A Beads (Promega, 50 μL slurry per sample) followed by capturing of the magnetic beads on the Andrew+ robot in a 96-well plate format. After washing, purified mAbs were eluted in 50 μL Glycine-HCl (2 M, pH 2.5) two times (100 μL total) and were added to a 60 μL neutralization buffer comprised of MES (100 mM) and Tris-HCl (900 mM) at pH 7.5.
Afterward, an aliquot of 20 μL purified mAbs (with <0.5 μg/μL concentration) was digested and the disulfide bonds were reduced to yield three mAb subunits (Fc/2, light chain, and Fd’) by adding 10 μL FabRICATOR (Ides) at 2 units/μL and 30 μL dithiothreitol (DTT) at 40 mM to each sample and incubating for 60 minutes at 37 °C.
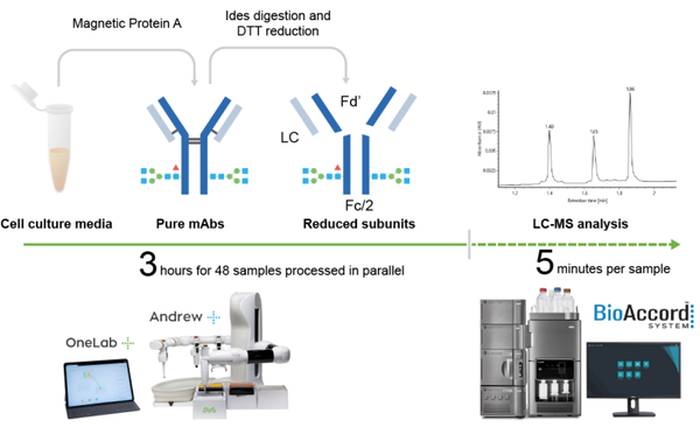
Figure 1: Workflow of automated antibody purification and digestion with Andrew+ and rapid LC-MS analysis using Waters BioAccord system.
Andrew+ Automation
Andrew+ provides a streamlined fully automated protocol for protein purification and digestion (Figure 2). In comparison, other automated liquid handlers might provide a semi-automated protocol that requires one to five manual intervention steps for a similar procedure.
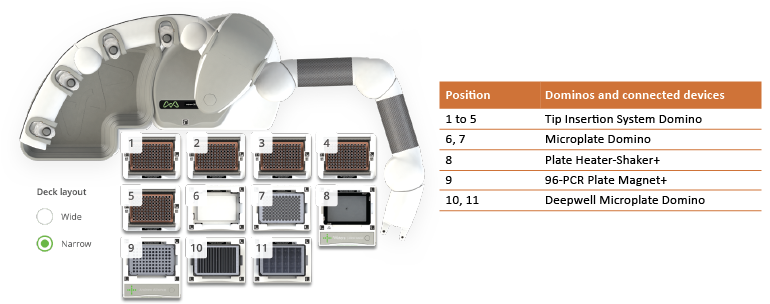
Figure 2: Andrew+ Dominos and connected devices configuration for the automated subunit analysis of 48 samples.
LC-MS Analysis
The LC-MS analysis of the mAb subunits was performed using 4.5 minutes, reversed-phase LC-MS method with 0.1% formic acid and acetonitrile mobile phase on a Waters™ BioAccord™ system.
Results and Discussion
High-throughput sample preparation using low volumes (20-100 μL) of cell culture media for purification and digestion was performed in a 96-well plate format by the Andrew+ pipetting robot. Purification of mAbs from crude samples such as cell culture media is a standard procedure during the process of development and manufacturing of therapeutic antibodies. It is usually performed using an affinity ligand such as Protein A, which binds specifically to IgG (Figure 3A). Protein A purification can be a tedious process as it involves numerous equilibrations and washing steps and entails a significant amount of manual labor. At the intact level, both non-purified and purified samples generated comparable results. The Protein A-purified samples resulted in higher quality chromatograms and were very similar to intact mAbs in buffer (Figure 3B).
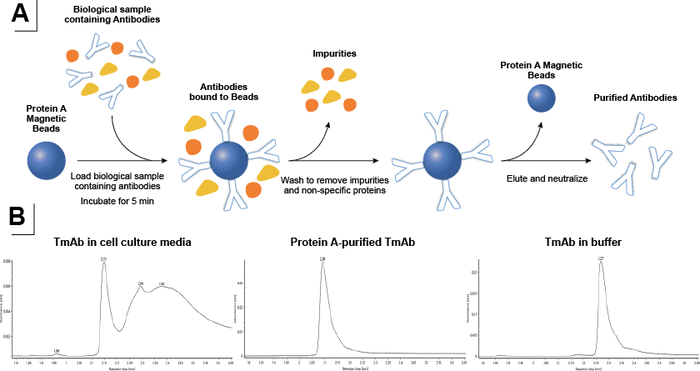
Figure 3: A) Schematic of antibody purification using Protein A-coupled magnetic beads. B) Chromatograms of TmAb in cell culture media (bottom left), after Protein A purification (bottom middle), and TmAb in buffer (bottom right).
A highly reproducible, scalable, and fully automated protocol was designed to be used with cell culture media samples using the Andrew+ pipetting robot. Samples were incubated with magnetic Protein A on the Shaker+ device followed by magnetic capture of the protein A beads to allow for removal of the flow-through fractions (Figure 3A). The magnetic Protein A beads with the captured mAbs were washed, and pure mAbs were eluted by lowering the pH. After neutralization of the pH, the pure mAb samples were digested using FabRICATOR (Ides) in the presence of a disulfide-bond reducing agent. This yielded reduced mAb subunits that were suitable for direct transfer into the autosampler of a Waters™ BioAccord™ system. Analysis of different fractions demonstrated effective capture of the mAbs from cell culture media, followed by elution of pure mAbs and digestion into subunits using FabRICATOR (Ides) and DTT (Figure 4).
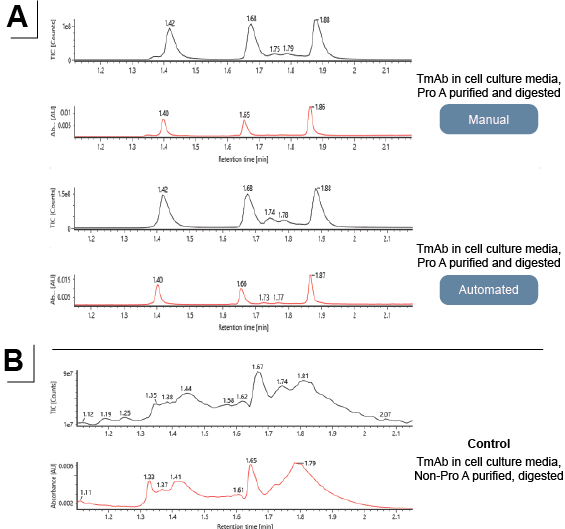
Figure 4: LC-MS analysis of subunits from (A) purified and digested mAbs, manual execution vs automated, and (B) from non-purified and digested mAbs.
The resulting reduced subunits were analyzed by reversed-phase LC-MS. Waters mAb subunit standard was used and the mass accuracy of the RDa™ mass detector for the subunit analysis was assessed as <20 ppm. The analysis of purified samples yielded high-quality spectra of all three subunits (Figure 5A), allowing for further analysis and quantification of different CQAs such as Fc glycosylation and glycation. Non-purified samples did not yield subunits suitable for LC-MS analysis. This can be due to inefficient enzymatic reaction in non-purified cell culture media and the presence of host cell proteins.
The LC-MS results of Protein A-purified and digested mAbs generated by both manual and Andrew+ were similar and identical to those from the control digestion (Figure 5B). The subunit level analysis more readily interpreted results for LC, Fd’, and Fc/2, and also provided additional information on scFc glycosylation.
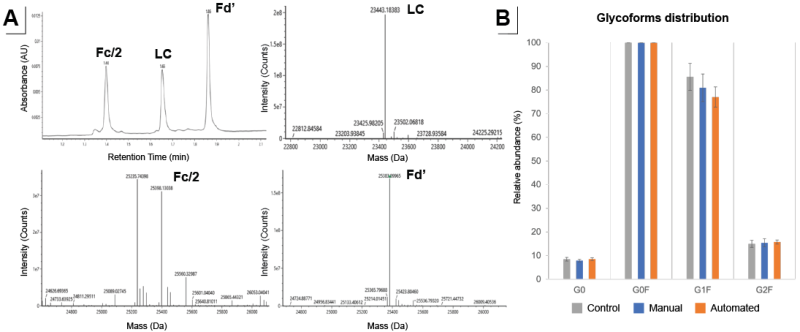
Figure 5: A) Subunit level analysis interpreted results for LC, Fd’, and Fc/2. B) Fc glycosylation profile from the deconvoluted mass spectrum of the Fc/2 fragment generated by manual (blue) and Andrew+ assisted (orange) compared to control (grey). N=8 for each condition.
Conclusion
Analytical process development is challenging as fast and robust analytics are crucial. With rapid automated sample preparation and LC-MS screening, with results comparable to the manual execution of the same protocol, mAbs can be analyzed directly from complex media for the determination of several important product quality attributes.
The fully automated combined protocol for purification and digestion takes 2 hours for 8 samples and 3 hours for 48 samples, and can consistently and reliably generate results similar to the manual execution of the procedure.
Furthermore, the ease-of-use BioAccord LC-MS system and informatics solution can improve the speed and data processing capabilities, allowing automated data acquisition and processing for rapid mass confirmation of the three mAb subunits.
ORDERING INFORMATION
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
➤ Rapid Subunit Screening, 8 Samples – Andrew+
- 2x Tip Insertion System Domino | p/n 186009612
- Microtube Domino | p/n 186009601
- Deepwell Microplate Domino | p/n 186009597
- Plate Heater-Shaker+ w/ Low-Profile 96-PCR Plate Adaptor | p/n 176005079
- 96-PCR Plate Magnet+ | p/n 176004850
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 µL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 µL | p/n 186009607
- Sartorius, Optifit Low Retention Tips, 5-350 µL (x170) | p/n LH-L790352
➤ Rapid Subunit Screening, 48 Samples – Andrew+
- 5x Tip Insertion System Domino | p/n 186009612
- 2x Deepwell Microplate Domino | p/n 186009597
- 2x Microplate Domino | p/n 186009600
- Microplate Heater-Shaker+ w/ Low-Profile 96-PCR Plate Adaptor | p/n 176005079
- 96-PCR Plate Magnet+ | p/n 176004850
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Andrew Alliance Bluetooth Electronic Pipette, 8-channel 300 μL | p/n 186009607
- Sartorius, Optifit Low Retention Refill Tips, 5-350 μL (x 392) | p/n LH-L790352
Reagents & Chemicals
- Magne® Protein A Beads by Promega | p/n G8782
- FabRICATOR® (IdeS) by Genovis | p/n A0-FR1-008
- Pierce™ DTT (Dithiothreitol), No-Weigh™ Format by Thermo Scientific | p/n A39255
Recommended Consumables
- Agilent 6-column reagent reservoir by Agilent Technologies | p/n 201284-100
- Axygen® 12-well reservoir, 12-channel trough | p/n RES-MW12-HP
- Eppendorf twin.tec® 96-well skirted LoBind® PCR plate by Eppendorf | p/n 0030129555
- Eppendorf 1.5 mL Safe-Lock tube by Eppendorf | p/n 0030120086
1) Lu et al., Development of therapeutic antibodies for the treatment of diseases, Journal of Biomedical Science 2020 27:1
2) Jia Dong, et al., High-Throughput, Automated Protein A Purification Platform with Multiattribute LC−MS Analysis for Advanced Cell Culture Process Monitoring, Anal. Chem. 2016, 88, 8673−8679
3) Lindsay Morrison, et al., Direct LC-MS Characterization of Glycoform Distribution and Low Molecular Weight Impurities in mAb Process from Ambr® 15 Bioreactors, Sartorius Application Note, March 29, 2022
4) Elsa Wagner-Rousset, et al., Antibody-drug conjugate model fast characterization by LC-MS following IdeS proteolytic digestion, mAbs 6:1, 173–184
5) Kuhn, E.; Fabbami, L.; Heuvel, Z. V. D.; Murphy, S.; Jaffe, J. D.; Carr, S. A. Automation of the Multiplexed Peptide Immune-MRM-MS Workflow on Bravo AssayMAP Platform. Broad Institute, Cambridge, MA; Agilent Technologies, Santa Clara, CA.
Protocols



Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 