or start from open source methods. Learn more about OneLab softwareUse OneLab
Automated Antibody Purification
This basic method provides the core methodology for translating a workflow into OneLab-executable script(s) as an attempt to fully or semi-automate a specific procedure. It demonstrates the benefits of automation and highlights OneLab capabilities and best practices to promote solution adoption, helping transition from manual to a more automated approach. It can be used alone or serves as a building block for a more complex workflow and is easily adaptable to users' requirements.
Overview
Rapid Automated Antibody Purification Protocol is Essential to the Development of Biotherapeutics
Monoclonal antibodies remain one of the fastest-growing classes of therapeutics. High-throughput small-scale analytical platforms are increasingly sought after within general research and to aid in the development of biopharmaceuticals. During process development, manufacturing, and quality control (QC) of therapeutic antibodies, purification is routinely carried out. Here, we present a rapid fully automated purification protocol that can extract antibodies in high yield from cell culture media. The protocol can be easily adjusted for both high and low titer samples to yield the purified antibodies at the desired concentration. More importantly, relatively low titer samples can be concentrated significantly to meet the needs of cell-based assays and protein characterization methods.
One-Step Affinity Purification Provides Antibody in High Yield
Cell culture media, prepared on the Ambr® 15 microbioreactor, was provided by Sartorius. For affinity purification, we employ magnetic beads with Protein A, covalently attached to the surface of the beads. Protein A provides a useful medium for routine antibody purification due to its strong and specific binding to the Fc region of many antibodies. In a single step, the high selectivity of Protein A produces a purified antibody in high yield.
Using magnetic beads for separation eliminates the need to use complex equipment such as centrifugation and filtration. This enables the full automation of protocol without the need for manual intervention.
The general principle behind the protocol for antibody purification with magnetic beads is illustrated below in Figure 1. Samples are first incubated with protein A magnetic beads in a binding buffer for a certain amount of time with gentle mixing. Then, impurities are washed away and bound antibodies are eluted from the beads using an acidic elution buffer.
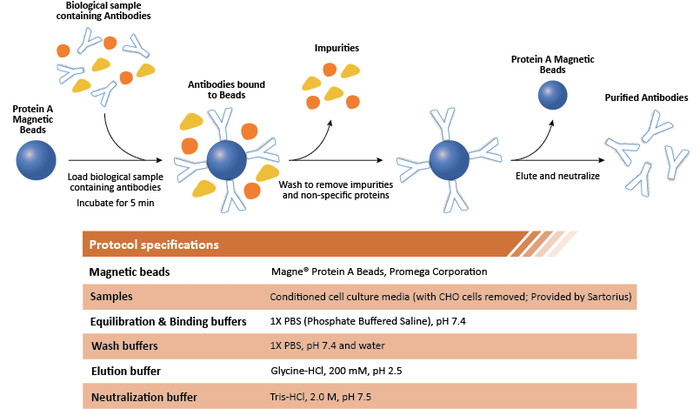
Figure 1: General protocol for antibody purification using Protein A-coupled magnetic beads.
Rapid Automated Antibody Purification Protocol Improves Sample Throughput
The general protocol is relatively simple, yet consists of several equilibration/washing steps, and manual execution can be tiresome. Using the fully automated protocol, Andrew+ can deliver a high yield of antibodies consistently and in a short amount of time. Our rapid automated protocol needs no manual interventions and the total execution time for 8 and 48 samples are 35 min (Figure 2) and 50 min (Figure 6), respectively. Andrew+ can effectively streamline the antibody purification with magnetic beads using the connected devices shown in Figure 2.
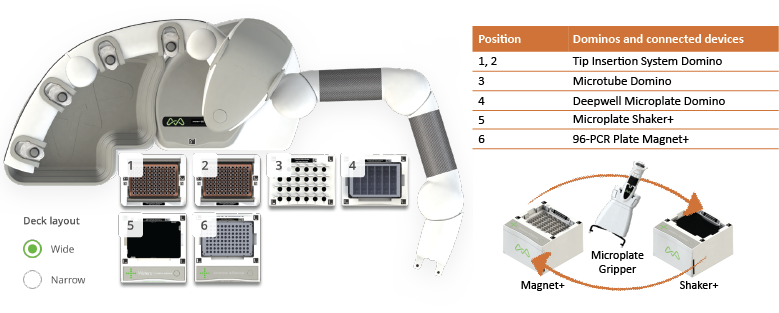
Figure 2: Configuration of Andrew+ Dominos and connected devices for the rapid automated antibody purification 8-sample protocol. For a fully automated protocol, Andrew+ requires two connected devices (Magnet+ and Shaker+) and a microplate gripper. The execution time for 8 samples is 35 min.
Rapid Automated Antibody Purification Protocol is Reproducible and Robust
Using the rapid automated protocol, Andrew+ can achieve high recovery of antibody (>70%) with reliable reproducibility (%RSD <10%). Recoveries obtained from the manual execution of the procedure and the automated procedure are comparable as shown in Figure 3.
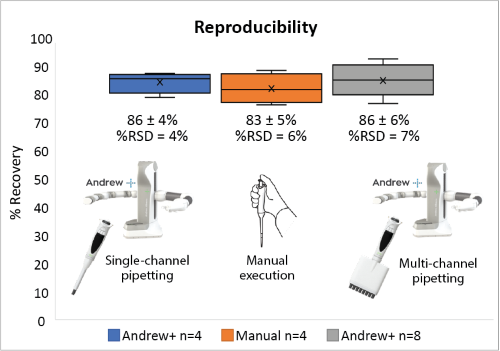
Figure 3: Comparison of the recovery of antibody obtained from the manual execution (n=4) and the Andrew+ Liquid Handling Robot (n=4 and n=8). Markers (x) represent the average values.
The robustness of the automated protocol was demonstrated by varying the amount of Protein A beads used for the capture step (Figure 4A) and the pH of the elution buffer (Figure 4B). The use of magnetic beads requires pipetting the beads from a homogenous slurry. To evaluate the impact of the amount of beads used on the recovery of antibodies, the standard amount of slurry used (50 μL) was varied by ±10%. These experiments with variations still produced satisfactory recovery of antibodies (>50%, Figure 4A).
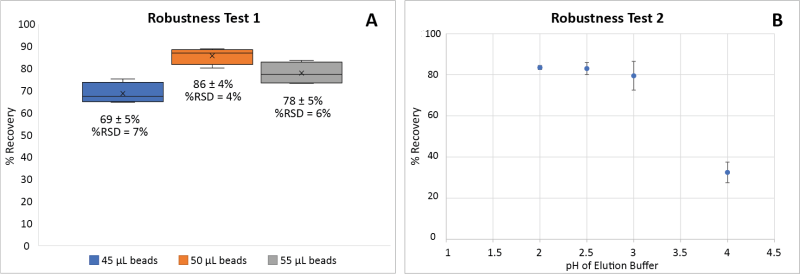
Figure 4: Robustness assessment of the automated protocol. Comparison of the recovery of antibody [A] with three different amounts of beads 45 μL, 50 μL (standard), and 55 μL (n=4); [B] with four different buffered pH levels pH = 2, 2.5 (standard), 3, and 4 (n=2). Markers (x) represent the average values.
Another variable that may have a significant impact on antibody recovery is the pH of the elution buffer. Our standard protocol uses Glycine-HCl (0.2 M) at pH 2.5 as the elution buffer. To test the robustness, elution buffers with a pH ranging from 2 to 4 were also tested. Recovery was not affected between pH 2 to 3 whereas significantly less recovery was observed for pH 4 (Figure 4B). Therefore, the method is robust between pH 2 and 3.
Rapid Automated Protocol Can Be Used for Both High and Low Titer Samples
While the standard incubation time of 5 min is adequate for low titer samples, for samples with high titer (>75 μg), a longer incubation time of 10 min is necessary to obtain a high recovery. The purification of samples with a titer of 150 μg results in 65% recovery with a 5 min incubation. Prolonging the incubation time from 5 min to 10 min increases the yield from 65% to 87%, as shown in Figure 5.
For samples with low titer (≤20 μg), multiple loadings can be performed to achieve a highly concentrated purified antibody. Three 100 μL loadings (5 min each) of cell culture media with a titer of 0.6 μg/μL (total of 180 μg) with an alternating washing step, yielded a comparable recovery of antibody (80%) to that of single loading (Figure 5).
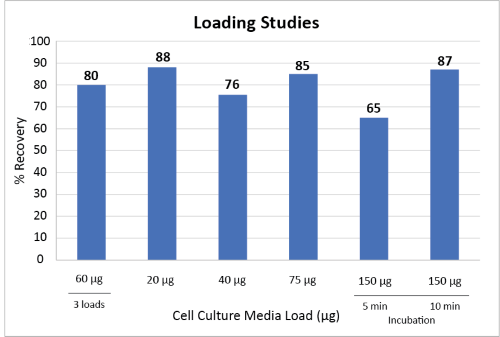
Figure 5: Antibody recoveries from cell culture media samples with various titers (20 μg, 40 μg, 75 μg, 150 μg, 180 μg). Multiple loadings were obtained by loading 60 μg three times with an intermediate washing step (n=2). Loading volumes were 100 μL. Titers for media samples ranged from 0.2 μg/μL to 1.5 μg/μL.
If samples of various concentrations are to be purified simultaneously, the “Concentration Normalization” feature on Andrew+ can be integrated prior to purification in order to normalize all samples to one concentration. Details on this normalization feature can be found in the OneLab Help Center.
Rapid Automated Protocol can be Used for High-Volume Applications
The automated protocol of 48 samples is described here for higher throughput applications such as clone selection. The sample throughput of 48 is suitable to be used in parallel with microbioreactors. The execution time of the automated protocol is 50 min and the Andrew+ domino configuration is shown below in Figure 6.

Figure 6: Configuration of Andrew+ Dominos and connected devices for the rapid automated antibody purification 48-sample protocol. For a fully automated protocol, Andrew+ requires two connected devices (Magnet+ and Shaker+) and a microplate gripper. The execution time for 48 samples is 50 min.
Considerations
1- Sample Collection Labware
- For method development applications, current protocols for 4, 8, and 48 samples save the flow-through solution to be analyzed further for cell culture media analysis. Alternatively, the protocol can be modified so that the flow-through samples are discarded into waste.
- The current protocol has set the eluted samples to be collected in the different wells of the same plate used for the Protein A purification in order to conserve labware. Alternatively, the protocol can be readily modified so that eluted samples can be collected into a separate collection plate.
2- Distribution of Magnetic Beads
- For 4 and 8-sample protocols, it is recommended to have 0.8-1 mL of magnetic beads slurry in the source tube to enable thorough mixing of the slurry by Andrew+ and the equal distribution of magnetic beads into sample wells.
3- Ensuring Complete Binding
- It is imperative that during the binding steps, beads must remain in suspension. Sample shaker speed may need to be adjusted if a different magnetic bead supplier is used.
ORDERING INFORMATION
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
➤ Rapid Automated Antibody Purification, 4 Samples – Andrew+
- 1x Tip Insertion System Domino | p/n 186009612
- Microtube Domino | p/n 186009601
- Deepwell Microplate Domino | p/n 186009597
- Microplate Shaker+ | p/n 176004577
- 96-PCR Plate Magnet+ | p/n 176004850
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Sartorius, Optifit Tips, 5-350 μL (x57) | p/n 700013297
➤ Rapid Automated Antibody Purification, 8 Samples – Andrew+
- 2x Tip Insertion System Domino | p/n 186009612
- Microtube Domino | p/n 186009601
- Deepwell Microplate Domino | p/n 186009597
- Microplate Shaker+ | p/n 176004577
- 96-PCR Plate Magnet+ | p/n 176004850
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Sartorius, Optifit Tips, 5-350 μL (x145) | p/n 700013297
➤ Rapid Automated Antibody Purification, 48 Samples – Andrew+
- 2x Tip Insertion System Domino | p/n 186009612
- Deepwell Microplate Domino | p/n 186009597
- Microplate Domino | p/n 186009600
- Microplate Shaker+ | p/n 176004577
- 96-PCR Plate Magnet+ | p/n 176004850
- 8-channel pipette reservoir Domino | p/n 186009613
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Sartorius, Optifit Tips, 5-350 μL (x168) | p/n 700013297
Reagents & Chemicals
- Magne® Protein A Beads by Promega | p/n G8782
Recommended Consumables
- Agilent 6-column reagent reservoir | p/n 201284-100
- Eppendorf twin.tec® 96-well skirted LoBind® PCR plate | p/n 0030129555
- Eppendorf 1.5 mL Safe-Lock tube | p/n 0030120086
- INTEGRA 10 mL multichannel reservoir | p/n 4332
1) Goyon A., D’Atri, V., Guillarme D. et al. Protocols for the analytical characterization of therapeutic monoclonal antibodies. J. Chrom. B. 2017; 1058, 73-84.
2) GE Healthcare. Antibody Purification Handbook
3) Choe, W., Durgannavar, TA., Chung, SJ. Fc-Binding Ligands of Immunoglobulin G: An Overview of High Affinity Proteins and Peptides. Materials 2016, 9, 994.
4) MagneTM Protein A Beads and MagneTM Protein G Beads for Antibody Purification. Promega Corporation: Madison, WI, 2015.
5) Bratz, M., Godat, B., Wieczorek, D., Nath, N. A Robist High-Throughput Method for Antibody Purification using Magnetic Beads on the KingFisher® Flex Platform. Promega Corporation: Madison, WI, 2016.
Discover Multi-Parallel Bioreactors by Sartorius for Cell Culture Media and Process Development
Protocols

Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 