or start from open source methods. Learn more about OneLab softwareUse OneLab
Analysis of mAbs in Crude Samples

This advanced method offers an end-to-end solution developed around an advanced application, a specialized kit, or an analytical system. It delivers complete functional, ready-to-use protocols that are qualitatively and quantitatively assessed for consistency, executability, and repeatability, usually without needing to make any changes. It operates per batch size and is scalable to accommodate changing requirements.
Overview
Towards Middle-Level Analysis for Characterization of Therapeutic mAbs
The development and manufacturing of therapeutic mAbs require analysis of many different critical quality attributes (CQAs), including Fc glycosylation, oxidation, C-terminal lysine clipping, and glycation. High-volume applications such as clone selection, process development, and stability studies generate numerous samples to be analyzed. This may create a bottleneck that can slow down the development of new therapeutics. There is a need for faster analytics with less manual labour during the sample preparation and analysis than the standard peptide mapping approaches allow for.
Analysis of mAbs at the intact level is fast and can be performed with little to no sample preparation, but it suffers from lower sensitivity and requires high-end mass spectrometry instrumentation often not readily available for routine analysis applications. The middle-level analysis offers a suitable alternative. It entails the generation of mAb subunits (23-25 kDa in size) by digesting the antibody at a specific site below the hinge using the FabRICATOR (IdeS) protease followed by reduction of the disulfide bonds. The resulting subunits are homogeneous and small enough to allow for the acquisition of high-quality spectra on most modern mass spectrometers with considerably shorter analysis time and simplified data analysis as compared to peptide mapping. Also, the middle-level analysis does not require prolonged, multi-step sample preparation, resulting in increased reproducibility and simplified automation of the process.
Here we present a fully automated workflow for the purification and digestion of therapeutic mAbs directly from complex samples such as harvested cell culture fluid (HCCF). It is based on purification using magnetic protein A beads followed by a combined digestion/reduction using FabRICATOR immobilized on magnetic beads (FabRICATOR® MAgIC, Genovis). The entire process is fully automated on the Andrew+ Pipetting Robot and yields reduced subunits ready for analysis by LC-MS (Figure 1).
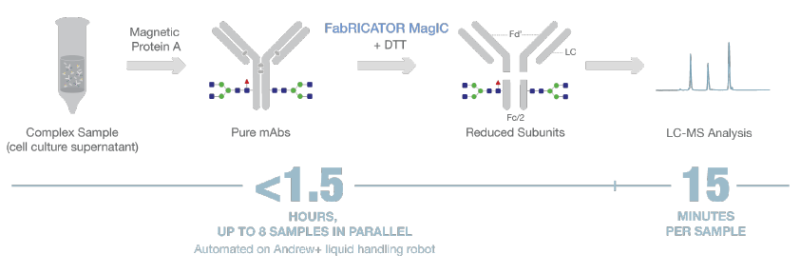
Figure 1: Workflow overview of antibody purification using magnetic protein A beads followed by FabRICATOR MagIC reaction that uses magnetic beads with immobilized IgG-specific FabRICATOR (IdeS) enzyme, a cysteine protease, to digest mAbs rapidly at a specific site below the hinge region, generating a homogenous pool of F(ab’)2 and Fc/2 fragments with minimal hands-on time. After digestion, samples can be directly transferred to the autosampler of the LC-MS for analysis. Using the Andrew+ robot, the magnetic bead format enables fast automated and consistent subunit generation of up to 8 samples in parallel for downstream analysis of CQAs in a middle-level LC/MS workflow.
Experimental Procedure
Purification of mAb samples from crude samples such as HCCF is a standard procedure during the development and manufacturing of therapeutic antibodies. It is usually performed using an affinity ligand such as Protein A, which binds specifically to IgG. Protein A purification can be a cumbersome process as it involves numerous equilibration and washing steps. For example, performing the purification process in a spin column format entails a significant amount of manual labour.
Thanks to the Magnet+ connected device, the Andrew+ Pipetting Robot is capable of handling reagents immobilized on magnetic beads. This allows for the automation of the protein A purification workflow by using magnetic Protein A beads. An overview of the domino configuration is shown in Figure 2. Samples were incubated with magnetic Protein A on the Shaker+ device followed by magnetic capture of the protein A beads to allow for the removal of the flow-through fractions while the mAbs are retained on the beads. The flow-through fractions were retained for optimization and control of the purification process as well as potentially spent medium analysis. The magnetic Protein A beads with the captured mAbs were washed, and pure mAbs were eluted by lowering the pH (100 mM glycine pH 2.5). After neutralization of the pH, the pure mAb samples were digested using FabRICATOR MagIC in the presence of 20 mM DTT. This yielded reduced mAb subunits which were collected in a microplate suitable for direct transfer to the autosampler of an LC-MS system. The FabRICATOR enzyme was retained in the reaction plate by the Magnet+ device and was thereby removed from the samples.
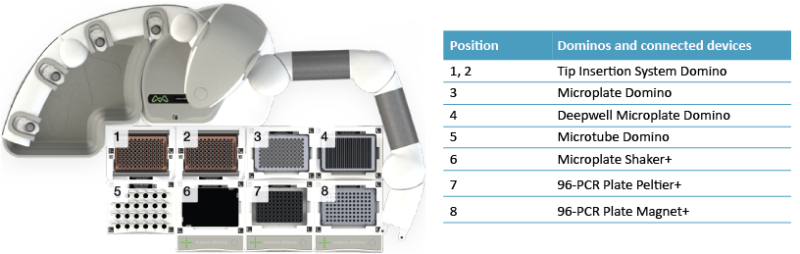
Figure 2: Domino/device positions on the Andrew+ working deck for the "IgG Purification + FabRICATOR MagIC digestion - 37°C" protocol.
Considerations
1- Depending on the available configuration, the digestion with FabRICATOR MagIC can either be performed at room temperature using the Shaker+ device or at 37°C on the Peltier+. Protocols for both setups are included.
2- The protocols for 4 samples use “dummy” samples in rows 5-8 to make use of the multichannel pipettes. This makes the protocol faster but leads to a somewhat higher consumption of pipette tips. If this is a concern, the protocol can be adapted for the use of a single-channel pipette.
3- The 350 µL 96-round well collection plate can be placed directly into the autosampler of a Waters LC-MS system. To that effect, the plate was closed using a polypropylene cap mat to avoid contamination and evaporation.
ORDERING INFORMATION
Andrew+ System Components: Dominos, Devices, Electronic Pipettes & Tips
➤ Automated FabRICATOR MagIC digestion, (4 Samples / 8 Samples) - 37°C
- Deepwell Microplate Domino | p/n 186009597
- Microplate Domino | p/n 186009600
- Microtube Domino | p/n 186009601
- Tip Insertion System Domino | p/n 186009612
- 96-PCR Plate Peltier+ | p/n 176004584
- 96-PCR Plate Magnet+ | p/n 176004850
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Sartorius, Optifit Tips, 5-350 μL (x71 for 4 Samples / x74 for 8 Samples) | p/n 700013297
➤ Automated FabRICATOR MagIC digestion, (4 Samples / 8 Samples) - RT
- Deepwell Microplate Domino | p/n 186009597
- Microplate Domino | p/n 186009600
- Microtube Domino | p/n 186009601
- Tip Insertion System Domino | p/n 186009612
- Microplate Shaker+ | p/n 176004577
- 96-PCR Plate Magnet+ | p/n 176004850
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Sartorius, Optifit Tips, 5-350 μL (x63 for 4 Samples / x66 for 8 Samples) | p/n 700013297
➤ IgG Purification + FabRICATOR MagIC digestion, (4 Samples / 8 Samples) - 37°C
- Deepwell Microplate Domino | p/n 186009597
- Microplate Domino | p/n 186009600
- Microtube Domino | p/n 186009601
- 2x Tip Insertion System Domino | p/n 186009612
- 96-PCR Plate Peltier+ | p/n 176004584
- Microplate Shaker+ | p/n 176004577
- 96-PCR Plate Magnet+ | p/n 176004850
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Sartorius, Optifit Tips, 5-350 μL (x189 for 4 Samples / x188 for 8 Samples) | p/n 700013297
➤ IgG Purification + FabRICATOR MagIC digestion, (4 Samples / 8 Samples) - RT
- Deepwell Microplate Domino | p/n 186009597
- Microplate Domino | p/n 186009600
- Microtube Domino | p/n 186009601
- 2x Tip Insertion System Domino | p/n 186009612
- Microplate Shaker+ | p/n 176004577
- 96-PCR Plate Magnet+ | p/n 176004850
- Andrew Alliance Bluetooth Electronic Pipette, 1-ch 300 μL | p/n 186009606
- Andrew Alliance Bluetooth Electronic Pipette, 8-ch 300 μL | p/n 186009607
- Andrew Alliance Bluetooth Microplate Gripper | p/n 186009776
- Sartorius, Optifit Tips, 5-350 μL (x173 for 4 Samples / x180 for 8 Samples) | p/n 700013297
Reagents & Chemicals
- FabRICATOR® MagIC, 2 mL by Genovis | p/n A0-FRM-024
- Protein A Mag Sepharose® Xtra, 2 x 1 mL by Cytiva | p/n 28967056
- DTT (1,4 Dithiothreitol) by G-Biosciences | p/n 786-077 or equivalent
- 1 M Tris, pH 8
- 100 mM Glycine, pH 2.5
- PBS (phosphate-buffered saline)
Recommended Consumables
- Fisherbrand™ Premium 1.5 mL microtube | p/n 11926955
- Eppendorf twin.tec® 96-well skirted LoBind® PCR plate | p/n 0030129555
- Axygen® 12-well reservoir, 12-channel trough | p/n RES-MW12-HP
- Waters 350 µL 96-round well collection plate | p/n 186002643
- Optional - Waters polypropylene cap mat for 96-well plate, round wells | p/n 186002483
Protocols




Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 