or start from open source methods. Learn more about OneLab softwareUse OneLab
ACE Inhibition Assay

This example method provides a freely adjustable framework for measuring the adaptability of the OneLab environment to workflows from different application fields. It helps to understand various nuances of the code-free, universal protocol designer and provides general indications as to the feasibility of a project. Scripts generally require fine adjustment to correct for variables and support specific labware implementation.
Overview
Angiotensin-Converting Enzyme (ACE) and Hypertension
The Renin-Angiotensin system or RAS plays a pivotal role in the physiology of blood pressure and it is regulation. The dysfunction of RAS is responsible for common pathologies including hypertension, heart failure, and renal disease.
In the RAS pathway, the Angiotensinogen precursor, secreted by the liver, is cleaved by the Renin enzyme to produce the Angiotensin I peptide. The Angiotensin-converting enzyme (ACE) then converts the Angiotensin I to Angiotensin II, an effector hormone, that mediates several effects, including vasoconstriction, aldosterone synthesis, and renal sodium reabsorption, through the activation of angiotensin II receptors (e.g. AT1 for Angiotensin II type 1 receptor), leading to an increase in blood pressure. Additionally, ACE catalyzes the degradation and inactivation of bradykinin, a potent vasodilator. The widespread action of ACE makes it a potential target for the treatment of hypertension as well as renal and cardiovascular disorders.
ACE Inhibitors
ACE inhibitors have been the cornerstone of RAS inhibition for years and are widely used in the treatment of hypertension and heart failure. Many bioactive natural compounds derived from plants are investigated for their inhibitory activity against ACE. The inhibition of ACE responsible for the conversion of angiotensin I to angiotensin II attenuates the effects of angiotensin II on blood pressure and potentiate the hypotensive activity of bradykinin by blocking its degradation.
Conventional Method for Determining ACE Inhibitory Activity
The ACE inhibitory activity of a sample was traditionally measured in vitro using the hippuryl-histidyl-leucine (HHL) as an ACE-specific substrate, which is converted to hippuric acid (HA) by the action of ACE. After stopping the reaction, the HA is extracted using an organic solvent (ethyl acetate) and its absorbance is measured by UV-Visible spectrophotometry (1). Another approach involves the use of High-Performance Liquid Chromatography (HPLC) to separate HA from HHL and UV detection for quantitation of HA (2). However, these procedures are cumbersome and rely on the extraction of HA using organic solvent. The residual ethyl acetate following liquid/liquid extraction can affect the accuracy of the measurement.
ACE Inhibition Assay Using ACE Kit-WST
The ACE Kit-WST provides a simple, plate-based colorimetric method to screen and measure ACE inhibitory activity without organic extraction. It consists of determining the amount of 3-Hydroxybutyric acid (3HB) generated from the substrate 3-Hydryoxybutyryl-Gly-Gly-Gly (3HB-GGG) by the combined action of ACE and another enzymatic reaction (Figure 1). The absorbance of the resulting colored solution can be measured using a microplate reader.
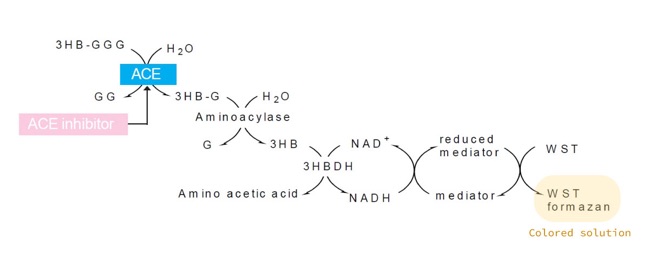
Figure 1: A schematic diagram of the enzymatic reaction allowing the detection of a potential ACE inhibitory activity.
Recommendations
For preparation of sample solutions, it is crucial to verify the following conditions to ensure proper measurement:
- If the sample is not water-soluble, use DMSO or ethanol to dissolve it. The final preparation should contain less than 1% DMSO or ethanol.
- If the sample is high in acidity due to the presence of citric acid or acetic acid, the content in the Substrate buffer will precipitate. The pH of the solution should be adjusted to 5 or above to ensure proper measurement.
- The presence of ascorbic acid in the sample may interfere with the assay by reducing the Indicator solution. To avoid any discrepancy, the final solution should contain less than 0.01% (w/v) ascorbic acid.
- If the sample solution contains insoluble material, it should be removed by centrifugation or filtration prior to use for the measurement.
(1) Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971 Jul;20(7):1637–48.
(2) Meng QC, Balcells E, Dell’Italia L, Durand J, Oparil S. Sensitive method for quantitation of angiotensin-converting enzyme (ACE) activity in tissue. Biochem Pharmacol. 1995 Oct 26;50(9):1445–50.
Learn more about the ACE Kit-WST and download complete protocols
Protocols


Contact info

 This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 